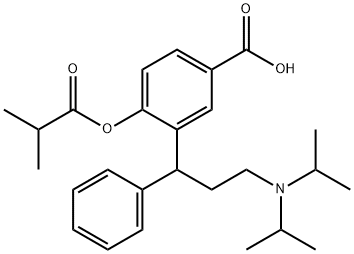
Fesoterodine Impurity 5 synthesis
- Product Name:Fesoterodine Impurity 5
- CAS Number:1262778-55-1
- Molecular formula:C26H35NO4
- Molecular Weight:425.56
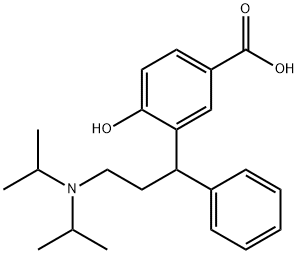
1076199-77-3
29 suppliers
inquiry
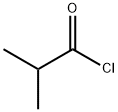
79-30-1
368 suppliers
$10.00/10g

1262778-55-1
16 suppliers
inquiry
Yield:1262778-55-1 100%
Reaction Conditions:
with triethylamine in dichloromethane at 25 - 30;
Steps:
2
EXAMPLE 2Obtaining 2- (3-N,N-diisopropylamine-l-phenylpropyl) -4- carboxyphenol isobutyrate (Va) Triethylamine (0.80 1, 2.05 equivalents) was loaded onto a suspension of the amino acid obtained in Example 1 (IVa) (1 kg) in dichloromethane (5 1) . Then, without the reaction temperature exceeding 25-300C, isobutyryl chloride (0.32 1, 1.1 equivalents) was loaded. The reaction conditions were maintained until the end of the reaction (1-2 hours) . The reaction mixture was cooled and a NH4Cl solution (10%) (5 1) was loaded, the pH being adjusted to 7. The product was concentrated to dryness, obtaining a residue with a purity greater than 85% and a molar yield of 95-100%.13NMR (1H) DMSO: Hi0: doublet 0.9-1.1 ppm 12H, H1: dd 1.2-1.3 ppm 6H, H7: multiplet 2.2-2.4 ppm 2H, H8: multiplet 2.6 ppm 2H, H2: heptuplet 2.9 ppm IH, H9: multiplet 3.2 ppm 2H, H6: triplet 4.2 ppm IH, H3: doublet 7.1 ppm IH, Hi3: multiplet 7.2 ppm IH, Hn-I2: multiplet 7.2-7.3 ppm 4H, H4: doublet 7.8 ppm IH, H5: singlet 8.1 ppm IH.NMR (13C) DMSO: 18.65; 18.79; 19.08; 19.25; 33.47; 34.22; 40.75;43.54; 50.14; 122.50; 126.37; 127.71; 128.27, 128.47; 128.98;132.55; 135.90; 143.19; 150.59; 167.80; 174.60.
References:
WO2011/12584,2011,A1 Location in patent:Page/Page column 26-27
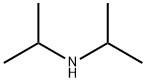
108-18-9
371 suppliers
$10.00/1g

1262778-55-1
16 suppliers
inquiry
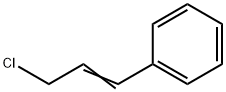
2687-12-9
165 suppliers
$5.00/5g

1262778-55-1
16 suppliers
inquiry