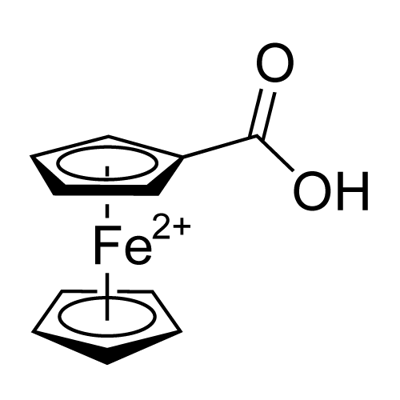
Ferrocenecarboxylic acid synthesis
- Product Name:Ferrocenecarboxylic acid
- CAS Number:1271-42-7
- Molecular formula:C11H10FeO210*
- Molecular Weight:230.04

102-54-5
462 suppliers
$5.00/25g
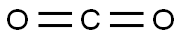
124-38-9
130 suppliers
$214.00/14L

1271-42-7
291 suppliers
$6.00/1g
Yield:1271-42-7 94%
Reaction Conditions:
Stage #1: ferrocenewith potassium tert-butylate;tert.-butyl lithium in tetrahydrofuran at -78; for 1 h;Inert atmosphere;Schlenk technique;
Stage #2: carbon dioxide in tetrahydrofuran at -78 - -15;Schlenk technique;
Steps:
Ferrocenecarboxylic Acid (3)
[CAS Reg. No. 1271-42-7] Ferrocene (29.8 g, 160 mmol, 1.00 equiv) and tBuOK (1.79 g, 16.0 mmol, 0.100 equiv) were introduced into a 2 L flame-dried roundbottomed flask, which was then subjected to three cycles of vacuum/ argon. Anhyd THF (1.23 L) was introduced by cannula and the reaction mixture was stirred at rt until dissolution of all solids. The mixture was cooled between -85 and -80 °C (external temperature) in an acetone/liquid N2 bath. tBuLi (1.6 M, 320 mmol, 200 mL, 2.00 equiv) was then introduced dropwise by a cannula keeping the temperature of the bath between -85 and -80 °C. After addition, the mixture was stirred at the same temperature for 1 h. Gaseous CO2 (dried by bubbling through concd H2SO4 and passing through an anhyd CaCl2 column) was bubbled into the reaction mixture for 30 min, keeping the temperature of the bath between -85 and -80 °C. The mixture was then allowed to warm to -15 °C, keeping the flask into the bath, with a continued bubbling of CO2. At -15 °C, bubbling of CO2 was stopped, the cooling bath was removed, and the mixture was warmed to rt. H2O (200 mL) was slowly added to the mixture, which was then extracted with 10% aq NaOH (6 150 mL). The combined aqueous layers were backwashed with Et2O (4 250 mL), cooled to 0 °C (ice-water bath), and acidified with HCl (35%) until pH 1 was reached. Caution: Vigorous evolution of CO2 occurred upon acidification. The resulting solids were filtered on a sintered-glass funnel (porosity 3), washed with H2O (5 250 mL) and pentane (1 250 mL), wringing the solid under vacuum between each wash. The resulting solid was dried overnight under high-vacuum (2 mbar) using P2O5 trap to give the title product as an orange solid (34.6 g, 94%); mp 208-210 °C (dec.); Rf = 0.30 (PE/EtOAc 75:25). Analytical data were analogous to those reported previously.24 IR (film): 2882 (br), 1651, 1474, 1282, 1158, 1029, 936, 914, 834, 740 cm-1. 1H NMR (500 MHz, DMSO-d6): =12.15 (br s, 1H, CO2H), 4.69 (s, 2 H, 2 FcCH), 4.43 (s, 2 H, 2 FcCH), 4.21 (s, 5 H, Cp). 13C NMR (126 MHz, DMSO-d6): = 172.1 (CO2H), 71.8 (FcC), 71.0 (2 FcCH), 69.9 (2 FcCH), 69.5 (Cp).
References:
Dorcet, Vincent;Erb, William;Roisnel, Thierry [Synthesis,2019,vol. 51,# 17,p. 3205 - 3213]
1271-55-2
223 suppliers
$8.00/1g

1271-42-7
291 suppliers
$6.00/1g

102-54-5
462 suppliers
$5.00/25g

1271-42-7
291 suppliers
$6.00/1g
1273-86-5
168 suppliers
$10.00/250mg

1271-42-7
291 suppliers
$6.00/1g
12093-10-6
188 suppliers
$8.00/1g

1271-42-7
291 suppliers
$6.00/1g