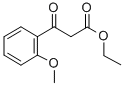
ETHYL (2-METHOXYBENZOYL)ACETATE synthesis
- Product Name:ETHYL (2-METHOXYBENZOYL)ACETATE
- CAS Number:41607-95-8
- Molecular formula:C12H14O4
- Molecular Weight:222.24
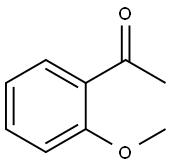
579-74-8
246 suppliers
$6.00/1g
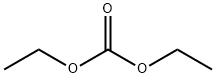
105-58-8
457 suppliers
$14.00/25g

41607-95-8
65 suppliers
$27.00/100mg
Yield: 88%
Reaction Conditions:
Stage #1:2-Methoxyacetophenone with sodium hydride in tetrahydrofuran at 0 - 20; for 0.5 h;Inert atmosphere;
Stage #2:Diethyl carbonate in tetrahydrofuran at 20; for 14 h;Inert atmosphere;
Steps:
6 4.5.5 Ethyl 3-(2-methoxyphenyl)-3-oxopropanoate (2c)
Sodium hydride (480mg, 20.0 mmmol) was taken in dry THF (18mL) in a 100mL round bottom flask under N2 and cooled it down to 0°C. To it was added a solution of ethyl 3-(2-methoxyphenyl)-3-oxopropanoate (1.0g, 6.7mmol) in THF (2mL). The reaction mixture was stirred at rt for 30min followed by the addition of diethyl carbonate (3.2mL, 26.8mmol). The reaction mixture was then stirred at rt for 14h. Ice-cooled water was added dropwise to quench the reaction. It was extracted with EtOAc (3×75mL). The combined organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude was further purified by flash chromatography on silica gel (60-120 mesh) using 10% hexane-EtOAc as eluent to afford 2c as a colourless liquid (1.3g, 88%). LC-MS (ESI) m/z 223.32 [M+H+]; 97% (purity).
References:
Zuniga, Edison S.;Korkegian, Aaron;Mullen, Steven;Hembre, Erik J.;Ornstein, Paul L.;Cortez, Guillermo;Biswas, Kallolmay;Kumar, Naresh;Cramer, Jeffrey;Masquelin, Thierry;Hipskind, Philip A.;Odingo, Joshua;Parish, Tanya [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 15,p. 3922 - 3946]

141-78-6
631 suppliers
$20.50/250ml
21615-34-9
278 suppliers
$12.00/25g

41607-95-8
65 suppliers
$27.00/100mg
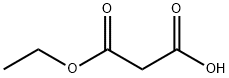
1071-46-1
291 suppliers
$5.00/1g

21615-34-9
278 suppliers
$12.00/25g

41607-95-8
65 suppliers
$27.00/100mg

623-73-4
149 suppliers
$26.69/5g
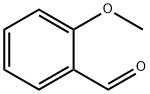
135-02-4
392 suppliers
$10.00/10g

41607-95-8
65 suppliers
$27.00/100mg
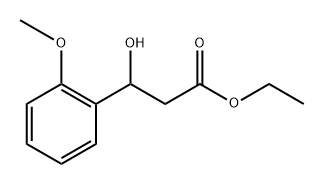
70200-18-9
4 suppliers
inquiry

41607-95-8
65 suppliers
$27.00/100mg