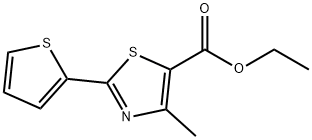
Ethyl4-Methyl-2-(thiophen-2-yl)thiazole-5-carboxylate synthesis
- Product Name:Ethyl4-Methyl-2-(thiophen-2-yl)thiazole-5-carboxylate
- CAS Number:56421-62-6
- Molecular formula:C11H11NO2S2
- Molecular Weight:253.34
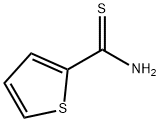
20300-02-1
126 suppliers
$24.00/1g
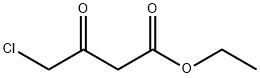
638-07-3
486 suppliers
$5.00/5 g

56421-62-6
46 suppliers
$63.00/500mg
Yield: 79%
Reaction Conditions:
in ethanol for 6 h;Reflux;
Steps:
General synthetic procedure forintermediates 13a-u
General procedure: To a solution of benzamide 11a-u (1 equiv) in THF (30mL) was added Lawesson’s reagent (0.6 equiv), and the mixture was heated to reflux for 4 hrs. The reaction mixture was concentrated invacuo,then diluted with ethyl acetate (30 ml), and washed with 1N NaHCO3 (3× 20 mL) and brine (2 × 20 mL). The organic layer was dried over anhydrous sodium sulfate,filtered and evaporated under reduced pressure. The crude product was purified by silica gel column chromatography using a mixture of dichloromethane/methanol(100:1, v/v) as eluent to afford a yellow solid product.A solution of the obtained solid 12a-u (1 equiv) and ethyl 2-chloroacetoacetate (1.2 equiv) in ethanol (25 ml) was heated to reflux for 6 h, then the mixture was allowed to stand at 0 °C for 10 hrs, and a white needle crystal was precipitate out.The reaction mixture was filtered and the filter cake was washed with 10 mL of ethanol, dried in vacuum to give the desired product.
References:
Li, Zheng;Qiu, Qianqian;Xu, Xue;Wang, Xuekun;Jiao, Lei;Su, Xin;Pan, Miaobo;Huang, Wenlong;Qian, Hai [European Journal of Medicinal Chemistry,2016,vol. 113,p. 246 - 257] Location in patent:supporting information
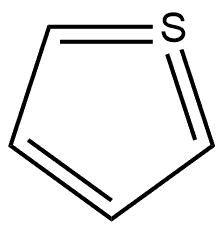
188290-36-0
1 suppliers
inquiry
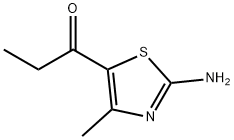
100289-17-6
5 suppliers
inquiry

56421-62-6
46 suppliers
$63.00/500mg
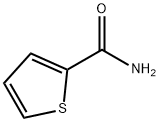
5813-89-8
93 suppliers
$14.00/1g

56421-62-6
46 suppliers
$63.00/500mg

20300-02-1
126 suppliers
$24.00/1g
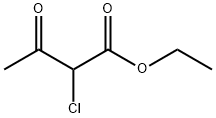
609-15-4
443 suppliers
$6.00/25g

56421-62-6
46 suppliers
$63.00/500mg