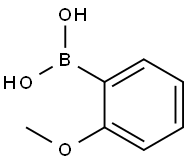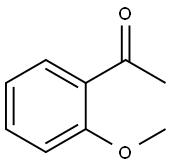
2'-Methoxyacetophenone synthesis
- Product Name:2'-Methoxyacetophenone
- CAS Number:579-74-8
- Molecular formula:C9H10O2
- Molecular Weight:150.17
Yield:579-74-8 86%
Reaction Conditions:
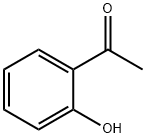
Stage #1:o-hydroxyacetophenone with lithium hydroxide monohydrate in tetrahydrofuran at 20; for 1 h;
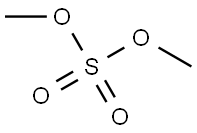
Stage #2:dimethyl sulfate in tetrahydrofuran for 60 h;
Steps:
4.1.5. 2-Methoxyphenyl methyl ketone 14
A solution of 1-(2-hydroxy-phenyl)-ethanone 13 (35.2 mL, 300 mmol) and LiOH·H2O (24.8 g, 590 mmol) in THF (400 mL) was stirred at room temperature for 1 h, and then dimethyl sulfate (42 mL, 293 mmol) was added. After completion of the reaction (TLC, 60 h), the solvent was removed under reduced pressure. The residue was dissolved in 2 M NaOH, and the resulting solution was extracted with diethyl ether. The combined extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to afford compound 14 (38.5 g, 256.0 mmol). Yield: 86%. 1H NMR (400 MHz, CDCl3): δ 7.71-7.69 (dd, J = 7.7, 1.8 Hz, 1H), 7.44-7.40 (m, 1H), 6.97-6.92 (m, 2H), 3.86 (s, 3H), 2.58 (s, 3H). 13C NMR (100.6 MHz, CDCl3): δ 199.8, 158.9, 133.7, 130.3, 128.2, 120.5, 111.6, 55.5, 31.8. IR (KBr): 3074, 3003, 2944, 2840, 1674, 1598, 1578, 1486, 1437, 1358, 1292, 1247, 1163, 1126, 1023, 967, 805, 758, 595, 534 cm-1.
References:
Dhondge, Attrimuni P.;Shaikh, Ajam C.;Chen, Chinpiao [Tetrahedron Asymmetry,2012,vol. 23,# 10,p. 723 - 733] Location in patent:experimental part
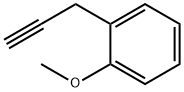
73234-87-4
1 suppliers
inquiry

579-74-8
245 suppliers
$6.00/1g
13513-82-1
58 suppliers
$11.00/250mg

579-74-8
245 suppliers
$6.00/1g
767-91-9
120 suppliers
$10.00/250mg

579-74-8
245 suppliers
$6.00/1g