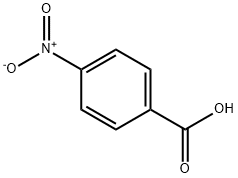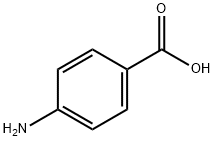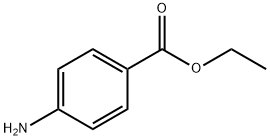
Benzocaine synthesis
- Product Name:Benzocaine
- CAS Number:94-09-7
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
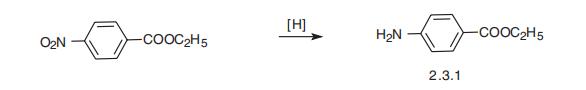
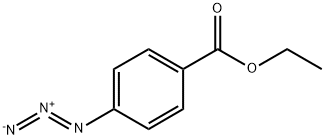
38556-93-3
1 suppliers
inquiry

94-09-7
777 suppliers
$5.00/50mg
Yield:94-09-7 100%
Reaction Conditions:
with hydrogen in methanol at 20; under 760.051 Torr; for 6 h;chemoselective reaction;Reagent/catalyst;
Steps:
4.4 General procedure for the hydrogenation
General procedure: A mixture of the substrate (0.250mmol) and catalyst [8% Pd/CR11 (3.3mg, 2.50μmol) or 9% Pd/CR20 (3.0mg, 2.50μmol)] in MeOH (1mL) was stirred under an H2 atmosphere (balloon) at room temperature. After a specific time, the mixture was filtered through a membrane filter (0.45 or 0.2μm), and the filtrate was concentrated in vacuo to give the corresponding spectrometrically-pure reduced product. If the reaction was not completed, the temperature was raised to 40 or 50°C. If the reaction was still incomplete, the H2 pressure was increased to 3 or 5atm. All the products were known, and the 1H NMR spectral data of the products were identical to those in the literature (see Supplementary data).
References:
Monguchi, Yasunari;Ichikawa, Tomohiro;Nozaki, Kei;Kihara, Kensuke;Yamada, Yuuko;Miyake, Yutaka;Sawama, Yoshinari;Sajiki, Hironao [Tetrahedron,2015,vol. 71,# 37,art. no. 26568,p. 6499 - 6505] Location in patent:supporting information
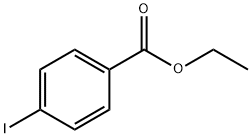
51934-41-9
286 suppliers
$6.00/5g

94-09-7
777 suppliers
$5.00/50mg
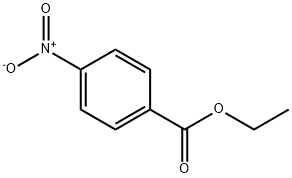
99-77-4
212 suppliers
$12.00/1mg

94-09-7
777 suppliers
$5.00/50mg