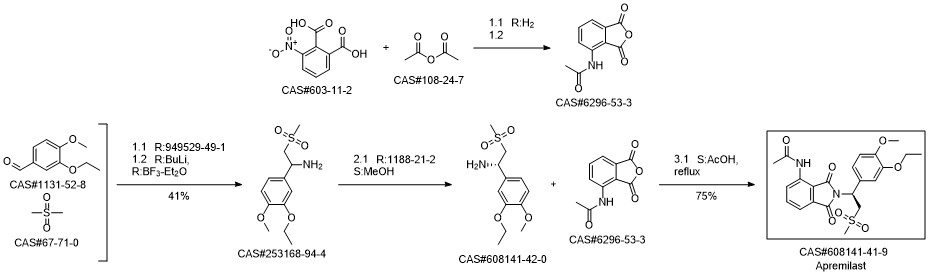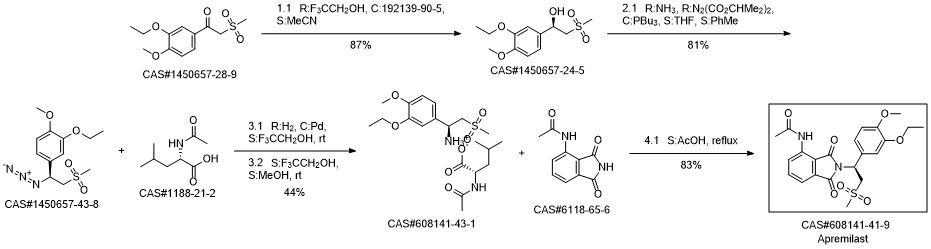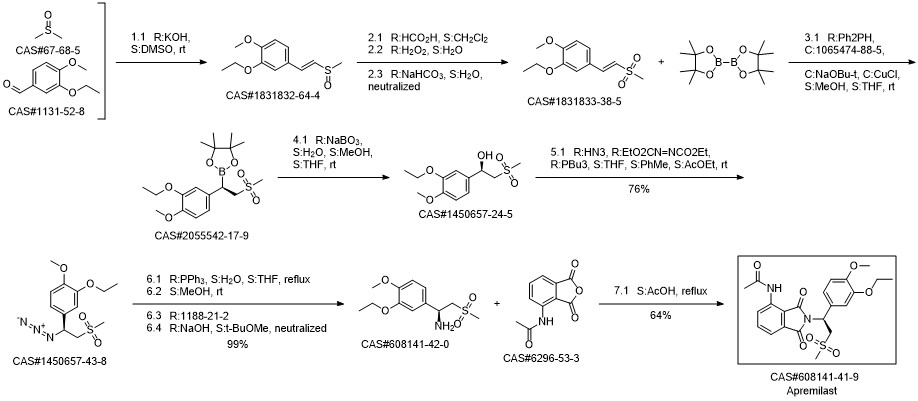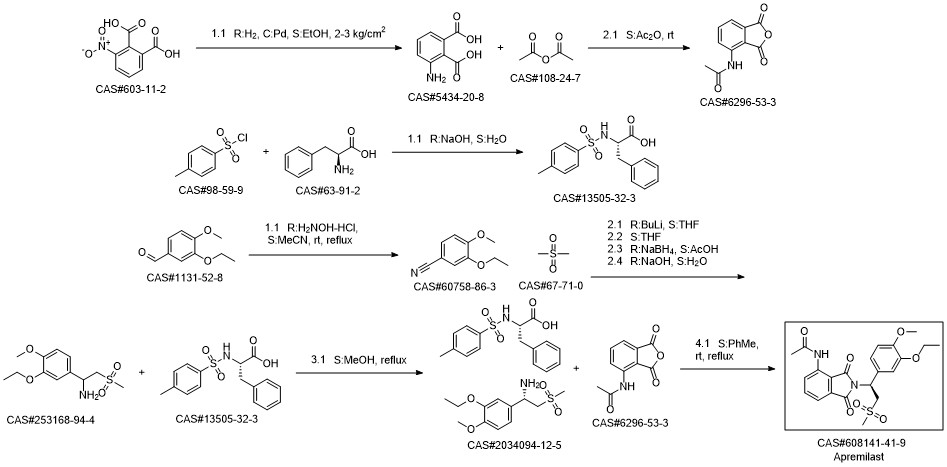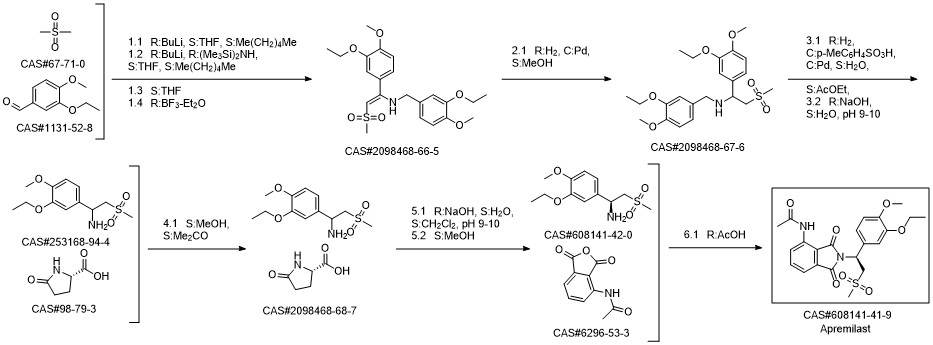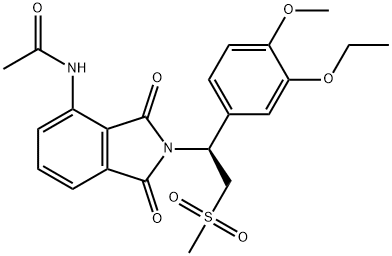
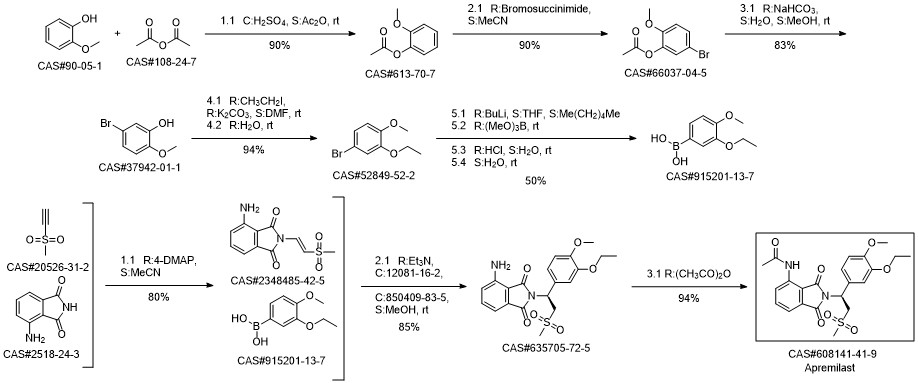
Apremilast synthesis
- Product Name:Apremilast
- CAS Number:608141-41-9
- Molecular formula:C22H24N2O7S
- Molecular Weight:460.5
Syu, Jin-Fong; Gopula, Balraj; Jian, Jia-Hong; Li, Wei-Sian; Kuo, Ting-Shen; Wu, Ping-Yu; Henschke, Julian P.; Hsieh, Meng-Chi; Tsai, Ming-Kang; Wu, Hsyueh-Liang. Asymmetric Synthesis of β-Aryl β-Imido Sulfones Using Rhodium Catalysts with Chiral Diene Ligands: Synthesis of Apremilast. Organic Letters. Volume 21. Issue 12. Pages 4614-4618. Journal; Online Computer File. (2019).
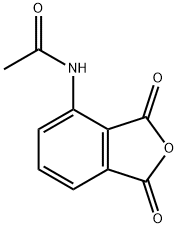
6296-53-3
304 suppliers
$6.00/250mg
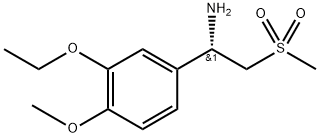
608141-42-0
224 suppliers
$10.00/250mg

608141-41-9
496 suppliers
$50.00/1g
Yield:608141-41-9 97%
Reaction Conditions:
with acetic acid for 1 h;Reflux;Large scale;
Steps:
3 Preparation of apremilast
The unbaked product (728 g after drying), glacial acetic acid (4. 2 L), (S)-1-(3-ethoxy-4-methoxyphenyl)-2-methanesulfonylethanamine(1570 g, 99.3%) Followed by adding the reaction flask, heating to reflux reaction for 1 hour, After the TLC was detected, the reaction was stopped and the reaction was terminated. The reaction was terminated under reduced pressure, and 10L of methylene chloride was dissolved. After washing with 5 L of water, 5 L of water saturated with aqueous sodium bicarbonate, 5 L of saturated sodium chloride, dried over anhydrous magnesium sulfate, Concentrated to dry, add anhydrous ethanol 10L, reflux 30 minutes, filtration, ethanol washing, 60 ° C drying to obtain product 1584g, the yield of 97%, HPLC 99. 8% or more, 99.2%.
References:
CN105294534,2016,A Location in patent:Paragraph 0030; 0031

6296-53-3
304 suppliers
$6.00/250mg
608141-43-1
183 suppliers
$18.00/1g

608141-41-9
496 suppliers
$50.00/1g

608141-42-0
224 suppliers
$10.00/250mg
![Benzoyl chloride, 4-[(2-chloroethyl)sulfonyl]-](/CAS/20180527/GIF/7185-00-4.gif)
7185-00-4
0 suppliers
inquiry

608141-41-9
496 suppliers
$50.00/1g