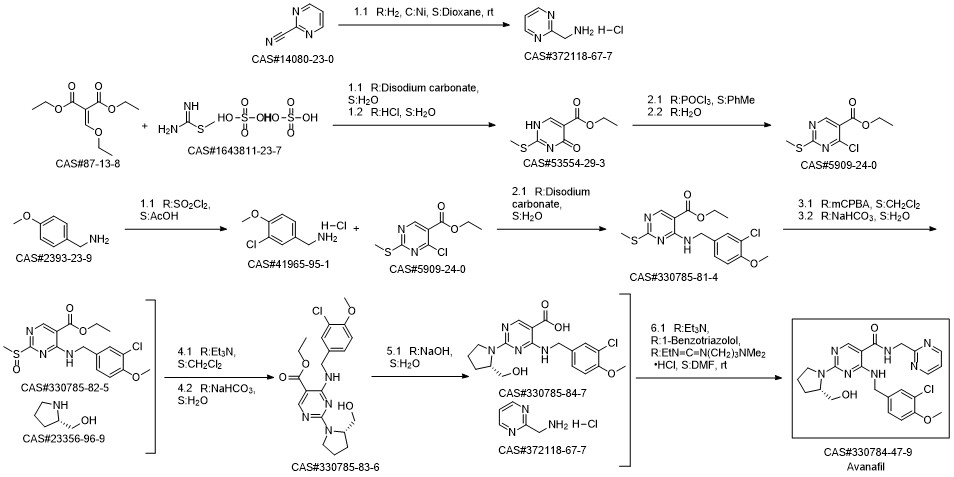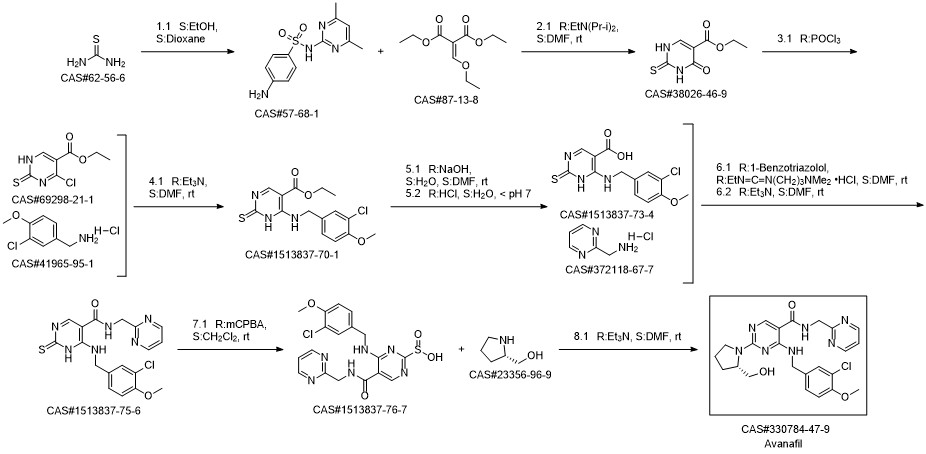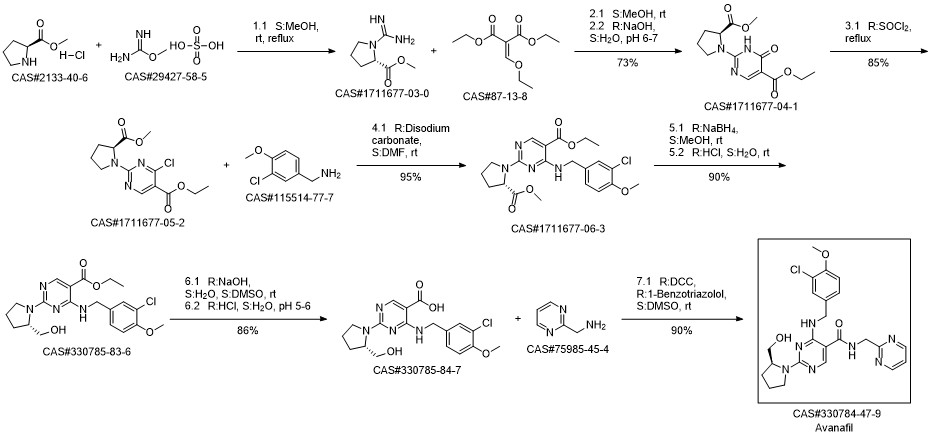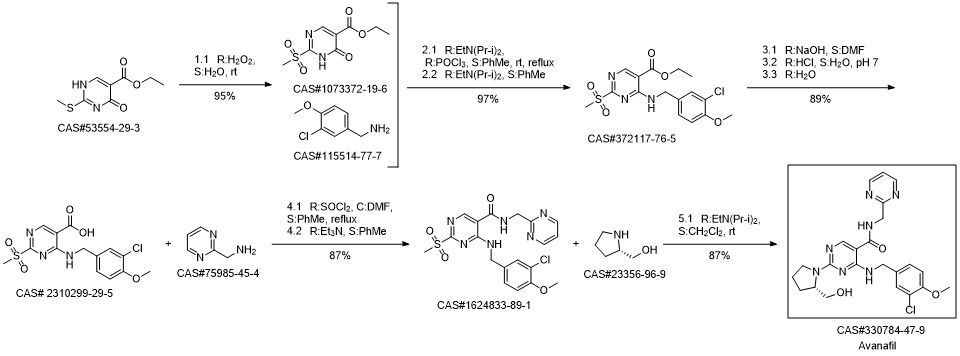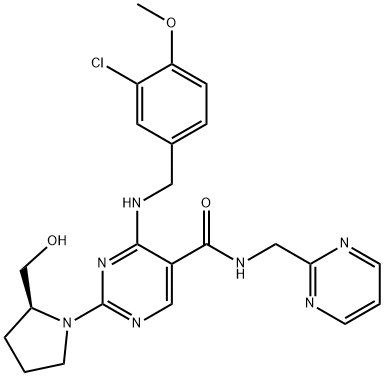
Avanafil synthesis
- Product Name:Avanafil
- CAS Number:330784-47-9
- Molecular formula:C23H26ClN7O3
- Molecular Weight:483.95
Sakamoto, Toshiaki; Koga, Yuichi; Hikota, Masataka; Matsuki, Kenji; Murakami, Michino; Kikkawa, Kohei; Fujishige, Kotomi; Kotera, Jun; Omori, Kenji; Morimoto, Hiroshi; Yamada, Koichiro. The discovery of avanafil for the treatment of erectile dysfunction: A novel pyrimidine-5-carboxamide derivative as a potent and highly selective phosphodiesterase 5 inhibitor. Bioorganic & Medicinal Chemistry Letters. Volume 24. Issue 23. Pages 5460-5465. Journal; Online Computer File. (2014).
![5-PyriMidinecarboxylic acid, 4-[[(3-chloro-4-Methoxyphenyl)Methyl]aMino]-2-[(2S)-2-(hydroxyMethyl)- 1-pyrrolidinyl]-](/CAS/GIF/330785-84-7.gif)
330785-84-7
122 suppliers
$80.00/250mg
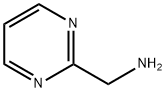
75985-45-4
159 suppliers
$10.00/250mg

330784-47-9
425 suppliers
$5.00/10mg
Yield:330784-47-9 99%
Reaction Conditions:
with dicyclohexyl-carbodiimide;1-hydroxy-1,2,3-benzotriazine-4(3H)-one in N,N-dimethyl-formamide at 0;Temperature;
Steps:
1 Preparation of avanafil
In the reaction flask, anhydrous DMF 3930 mL, compound 5 (393 g, 1.0 mol), 2-aminopyrimidine (163.5 g,1.5 mol), DCC (210 g, 1.02 mol) and 1-hydroxy-1,2,3-benzotriazine-4(3H)-one (166 g, 1.02 mol) at 0 ° CThe reaction was stirred, monitored until Compound 5 was completely reacted, filtered, and the filtrate was added to water, extracted with chloroform, washed with organic phase and dried.After the filtrate is too short, the silica gel layer is spin-dried to obtain crude avervavir, and the crude avervavir is purified by methanol to obtain pure avenue.The yield was 99.0% and the purity was 99.85%.
References:
CN109232542,2019,A Location in patent:Paragraph 0014; 0037-0038; 0048-0049; 0059-0060