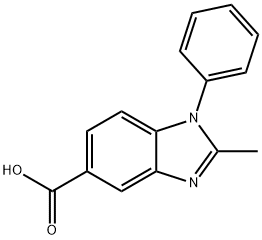
2-METHYL-1-PHENYL-1H-BENZOIMIDAZOLE-5-CARBOXYLIC ACID synthesis
- Product Name:2-METHYL-1-PHENYL-1H-BENZOIMIDAZOLE-5-CARBOXYLIC ACID
- CAS Number:92437-43-9
- Molecular formula:C15H12N2O2
- Molecular Weight:252.27
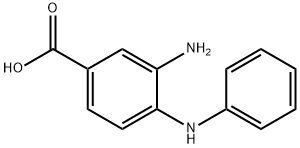
55296-17-8
11 suppliers
inquiry
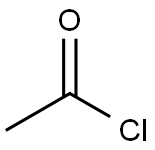
75-36-5
579 suppliers
$17.92/100G

92437-43-9
42 suppliers
$75.00/500mg
Yield:92437-43-9 83.5%
Reaction Conditions:
Stage #1: 3-amino-4-(phenylamino)benzoic acid;acetyl chloridewith hydrogenchloride in toluene; for 2.75 h;Reflux;
Stage #2: with sodium hydroxide in water;toluene at 20;
Stage #3: with hydrogenchloride in water at 5 - 10; pH=~ 4;
Steps:
1.1.3
Synthesis Example 1-3 Synthesis of 2-methyl-1-phenylbenzimidazole-5-carboxyhc acid A 2-neck flask was charged with 3-amino-4-phenylaminobenzoic acid (see Synthesis Example 1-2) (1.40 g, 6.35 mmol) and anhydrous toluene (15 mL) and this was refluxed. To this was added dropwise over approx.15 minutes acetyl chloride (1.00 g, 12.70 mmol) in toluene solution (approx. 2.5 mL), and this was stirred under these conditions for 2.5 hours. This was allowed to cool to room temperature, and was extracted with dilute aqueous sodium hydroxide solution (10%, 200 mL). After separating the dark orange aqueous layer, it was cooled in ice (5 to 10 °C), and concentrated hydrochloric acid (12 M) was added to the liquid to give approx. pH 4. The precipitated crystals were filtered off, washed with distilled water, and dried under reduced pressure with heating to yield the title compound (1.34 g, 83.5% yield) as a light purple solid. 1H-NMR (DMSO-d6) δ (ppm): 2.47 (s, 3H), 7.18 (d, 1H, J = 8.4 Hz), 7.58 (d, 2H, J = 7.0 Hz), 7.59 (dt, 1H, J = 9.1,1.5 Hz), 7.67 (t, 2H, J = 7.7 Hz), 7.83 (dd, 1H, J = 8.5,1.4 Hz), 8.21 (d, 1H, J =1.0 Hz), 12.75 (brs,1H).
References:
EP2436683,2012,A1 Location in patent:Page/Page column 44
62-53-3
658 suppliers
$10.00/1g

92437-43-9
42 suppliers
$75.00/500mg
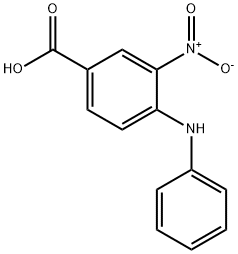
16927-49-4
15 suppliers
inquiry

92437-43-9
42 suppliers
$75.00/500mg
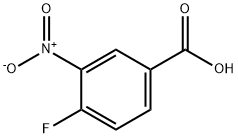
453-71-4
328 suppliers
$5.00/1g

92437-43-9
42 suppliers
$75.00/500mg