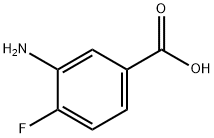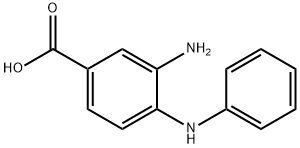
3-AMINO-4-PHENYLAMINO-BENZOIC ACID synthesis
- Product Name:3-AMINO-4-PHENYLAMINO-BENZOIC ACID
- CAS Number:55296-17-8
- Molecular formula:C13H12N2O2
- Molecular Weight:228.25
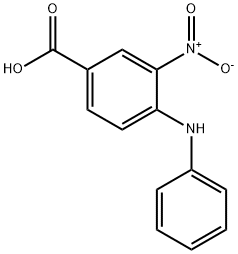
16927-49-4
15 suppliers
inquiry

55296-17-8
11 suppliers
inquiry
Yield:55296-17-8 95.3%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in tetrahydrofuran at 20; for 2.5 h;
Steps:
1.1.2
Synthesis Example 1-2 Synthesis of 3-amino-4-phenylaminobenzoic acid A 3-neck flask was charged with 3-mtro-4-phenylaminobenzoic acid (see Synthesis Example 1-1) (1.67 g, 6.70 mmol), palladium/ carbon (Pd:10%, z0 g), and tetrahydrofuran (34 mL), the atmosphere was replaced with hydrogen using three cycles of vacuum/hydrogen purge, and this was stirred at room temperature for 2.5 hours. After substituting in nitrogen, distilled water (20 mL) was added and this was stirred for 10 minutes, insoluble material was filtered off through a Celite layer (20 mm thickness), and this same layer was further washed with tetrahydrofuran (20 mL, 3 times). The filtrate and the wash solutions were combined, and the solvent was distilled off under reduced pressure. This was successively extracted with ethyl acetate (approx. 50 mL, 4 times), washed with distilled water (30 mL), and dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and further drying under reduced pressure yielded the title compound (1.45 g, 95.3% yield) as a pale yellow solid. 1H-NMR (DMSO-d6) δ (ppm): 4.98 (brs, 2H), 6.83 (dt, 1H, J = 7.4,1.0 Hz), 6.97 (dd, 2H, J = 7.6, 1.0 Hz), 7.06 (d, 1H, J = 8.3 Hz), 7.15 (dd, 1H, J = 8.2,1.9 Hz), 7.23 (t, 2H, J = 7.4 Hz), 7.33 (d, 1H, J = 1.9 Hz), 7.35 (s, 1H), 12.25 (brs, 1H).
References:
EP2436683,2012,A1 Location in patent:Page/Page column 44
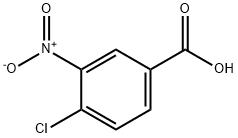
96-99-1
498 suppliers
$5.00/5g

55296-17-8
11 suppliers
inquiry
62-53-3
658 suppliers
$10.00/1g

55296-17-8
11 suppliers
inquiry
453-71-4
328 suppliers
$5.00/1g

55296-17-8
11 suppliers
inquiry