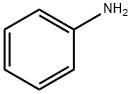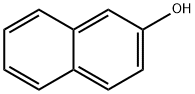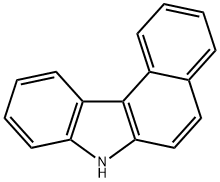
7H-BENZO[C]CARBAZOLE synthesis
- Product Name:7H-BENZO[C]CARBAZOLE
- CAS Number:205-25-4
- Molecular formula:C16H11N
- Molecular Weight:217.27
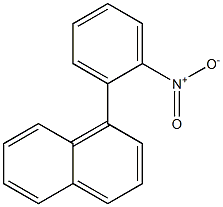
5415-59-8
5 suppliers
inquiry
![7H-BENZO[C]CARBAZOLE](/CAS/GIF/205-25-4.gif)
205-25-4
132 suppliers
$7.00/100mg
Yield:205-25-4 98%
Reaction Conditions:
with 1,10-Phenanthroline;sodium palladium(II) tetrachloride;formyloxybenzene;sodium salt of phosphorous acid in N,N-dimethyl-formamide at 170; for 5 h;Inert atmosphere;Schlenk technique;Sealed tube;
Steps:
2.3. General procedure for catalytic reactions
General procedure: The catalyst precursors were handled, weighed and stored inthe air except for Pd(OAc)2 that was stored under dinitrogen. Inorder to avoid weighing very small amounts of the catalysts, stocksolutions (under dinitrogen) of palladium catalysts were preparedin the appropriate solvent. For catalytic runs that required anamount of Phen less than 10 mg, a stock solution was preparedto avoid large weighing errors. In a typical reaction, the substratewas weighed in the air and placed in a 23 mL heavy-walled glasspressure tube with screw thread (Duran) containing a magneticstirring bar. (For an in-depth discussion of the different kinds ofpressure tubes that can be employed, and the correspondingadvantages and disadvantages see ref. [33]). The tube was placedin a Schlenk tube with a wide mouth. The tube was evacuatedand filled with dinitrogen three times to remove air. The catalystand ligand solutions and the amount of solvent required to reacha total volume of 10 mL were added and the reaction mixturewas stirred for 10 min to allow Phen to coordinate to palladium.Finally, HCOOPh and the base were added in this order. The pressuretube was plugged with a Schott PTB screw cap completed witha PTFE-protected seal and immersed in an oil bath preheated at theappropriate temperature. At the end of the reaction, the pressuretube was lifted from the oil and let to cool to room temperature.Then, the screw cap was carefully removed, the excess of CO wasvented, and the content was analyzed by GC or subjected to columnchromatography. (Caution: evolution of dissolved CO fromthe solution may continue for several minutes; keep the tubeunder a hood).
References:
Ferretti, Francesco;Ragaini, Fabio;Ramadan, Doaa R. [Journal of Catalysis,2022,vol. 409,p. 41 - 47]
![5H,6H,7H-benzo[c]carbazole](/CAS/GIF/5425-53-6.gif)
5425-53-6
4 suppliers
inquiry
![7H-BENZO[C]CARBAZOLE](/CAS/GIF/205-25-4.gif)
205-25-4
132 suppliers
$7.00/100mg
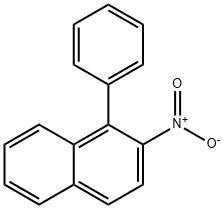
92795-22-7
0 suppliers
inquiry
![7H-BENZO[C]CARBAZOLE](/CAS/GIF/205-25-4.gif)
205-25-4
132 suppliers
$7.00/100mg
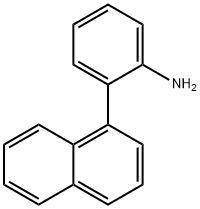
92855-12-4
11 suppliers
inquiry
![7H-BENZO[C]CARBAZOLE](/CAS/GIF/205-25-4.gif)
205-25-4
132 suppliers
$7.00/100mg