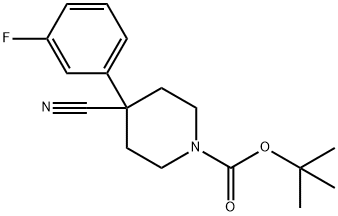
1-N-BOC-4-(3-FLUOROPHENYL)PIPERIDINE-4-CARBONITRILE synthesis
- Product Name:1-N-BOC-4-(3-FLUOROPHENYL)PIPERIDINE-4-CARBONITRILE
- CAS Number:619292-30-7
- Molecular formula:C17H21FN2O2
- Molecular Weight:304.36
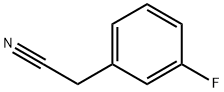
501-00-8
266 suppliers
$21.00/5g
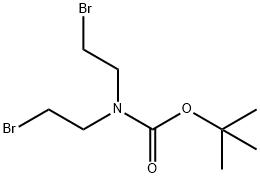
159635-50-4
67 suppliers
$45.00/50mg

619292-30-7
11 suppliers
inquiry
Yield:619292-30-7 86%
Reaction Conditions:
with potassium-t-butoxide in dimethyl sulfoxide at 0 - 20; for 17.5 h;
Steps:
7.P1
P1. Weigh 50 g of 3-fluorobenzeneacetonitrile into a three-necked flask, add 250 ml of dimethyl sulfoxide to dissolve, cool the solution to 0°C under ice-water bath conditions, and stir at a rate of 1000 r/min until the reaction ends, add Potassium tert-butoxide 87.2g, N-Boc-N,N-bis(2-bromoethyl)amine 122.5g was added dropwise at a rate of 1ml/min, the temperature was controlled to react at 5-10°C for 1.5h, and the ice-water bath was removed , heated at 20 °C, and the temperature was controlled at 20 °C for 16 h. After the reaction was completed, 600 ml of ice water was added to quench the reaction, and the reaction was extracted twice with ethyl acetate, using 250 ml of ethyl acetate for each extraction. The organic phases were combined, washed with saturated sodium chloride solution (20°C, saturated solution at standard atmospheric pressure), and the solvent was spun dry by rotary evaporator to give 1-Boc-4-cyano-4-(3-fluorophenyl) ) piperidine
References:
CN114213255,2022,A Location in patent:Paragraph 0050

501-00-8
266 suppliers
$21.00/5g
118753-70-1
176 suppliers
$6.00/1g

619292-30-7
11 suppliers
inquiry