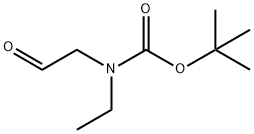
N-Boc-(ethylamino)acetaldehyde synthesis
- Product Name:N-Boc-(ethylamino)acetaldehyde
- CAS Number:315718-06-0
- Molecular formula:C9H17NO3
- Molecular Weight:187.24
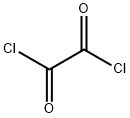
79-37-8
473 suppliers
$17.67/10gm:
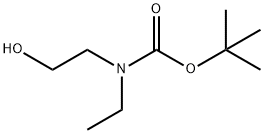
152192-95-5
64 suppliers
$26.00/1g

315718-06-0
44 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with triethylamine in methanol;dichloromethane;water;dimethyl sulfoxide;
Steps:
29.B Step B
Step B 2-(N-Tert-butyloxycarbonyl-N-ethyl)aminoacetaldehyde To a mixture of oxalyl chloride (3.4 ml of 2M solution in CH2Cl2, 6.87 mmol) in CH2Cl2 (10 ml) at -78° C. was added a solution of DMSO (0.86 ml, 12.14 mmol) in CH2Cl2 (5 ml) slowly. The mixture was stirred at -78° C. for 20 min. To this was added a solution of 2-(N-Tert-butyloxycarbonyl-N-ethyl)aminoethanol (1.0 g, 5.28 mmol) in CH2Cl2 (5 ml) slowly. The reaction mixture was then stirred at -78° C. for 2 h followed by addition of triethylamine (3.7 ml, 26.4 mmol). The mixture was stirred again at -78° C. for 10 min, at 0° C. for 30 min. A mixture of methanol (1 ml) and water (8 ml) was added to the reaction; the mixture was separated. The aqueous layer was extracted with methylene chloride (3*60 ml). The combined organic layer was washed with saturated sodium bicarbonate, water, brine and dried over magnesium sulfate. Removal of the solvent provided the title compound, which was used directly for next step.
References:
US6316444,2001,B1

24424-99-5
835 suppliers
$13.50/25G

315718-06-0
44 suppliers
$45.00/50mg

152192-95-5
64 suppliers
$26.00/1g

315718-06-0
44 suppliers
$45.00/50mg