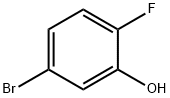
5-Bromo-2-fluorophenol synthesis
- Product Name:5-Bromo-2-fluorophenol
- CAS Number:112204-58-7
- Molecular formula:C6H4BrFO
- Molecular Weight:191
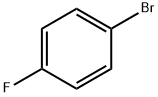
460-00-4
620 suppliers
$6.00/10g

112204-58-7
213 suppliers
$10.00/5g
Yield:112204-58-7 85%
Reaction Conditions:
Stage #1:1-Bromo-4-fluorobenzene with 2,2,6,6-tetramethyl-piperidine;n-butyllithium in tetrahydrofuran;hexane at -78;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexane at -78 - 20; for 2.5 h;
Steps:
7A
To a solution of 2,2,6,6-tetramethyl piperidine (5.6 mL, 33.2 mmol) in THF at -20° C. was added n-BuLi (1.6 M in hexanes, 18.8 mL, 30 mmol). The mixture was stirred at -20° C. for 10 min before it was cooled to -78° C. 1-Bromo-4-fluorobenzene (2.95 mL, 27 mmol) was added over 10 min and the mixture was stirred at -78° C. for 2.0 h before trimethyl borate (6.0 mL, 54 mmol) was added. The mixture was stirred at -78° C. for 30 min and then at rt for 2.0 h. After it was cooled back to 0° C., glacial acetic acid (4.86 mL, 81 mmol) was added and stirred for 30 min, followed by addition of 30% H2O2 (4.86 mL, 81 mmol). The mixture was stirred at rt for 24 h, quenched by addition of MnO2 (40 mg). After stirring at rt for 30 min, the cloudy solution was filtered through a pad of wet Celite and extracted with EtOAc. The EtOAc layer was washed with aquous NaHSO3, brine and dried over Na2SO4. The crude residue was purified by flash column chromatography (EtOAc:hexanes=1:5) to give 4.4 g (85%) of 7A as a liquid. 1H NMR (400 MHz, CDCl3) δ ppm 5.39 (s, 1H) 6.90-6.98 (m, 2H) 7.14 (dd, J=8.13, 1.98 Hz, 1H).
References:
Bristol-Myers Squibb Company US2010/227894, 2010, A1 Location in patent:Page/Page column 27
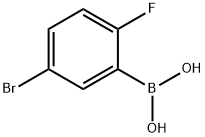
112204-57-6
126 suppliers
$11.00/1g

112204-58-7
213 suppliers
$10.00/5g
103291-07-2
126 suppliers
$10.00/5g

112204-58-7
213 suppliers
$10.00/5g
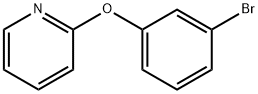
92545-83-0
21 suppliers
$106.00/250 mg

112204-58-7
213 suppliers
$10.00/5g
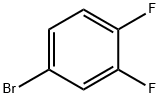
348-61-8
390 suppliers
$6.00/5g
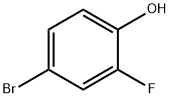
2105-94-4
350 suppliers
$6.00/5g

112204-58-7
213 suppliers
$10.00/5g