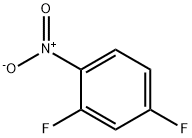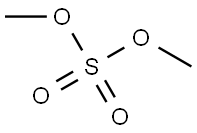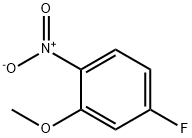
5-Fluoro-2-nitroanisole synthesis
- Product Name:5-Fluoro-2-nitroanisole
- CAS Number:448-19-1
- Molecular formula:C7H6FNO3
- Molecular Weight:171.13
Yield:448-19-1 87.38%
Reaction Conditions:
with potassium tert-butylate in toluene at 0 - 20;
Steps:
1.A Step-A- process for the preparation of 4-fluoro-2-methoxy-l-nitrobenzene:
Toluene (500 ml) was added into clean and dry round bottom flask, then 2,4- difluoro-1 -nitrobenzene (500 gms) was added. The reaction mass was cooled to 0°C then methanol (100 ml) was slowly added to reaction mass at 0°C. To the reaction mass potassium tert-butoxide (PTB) (353 gms) was added in lots (10 lots) at 0°C. The reaction mass was stirred at 0°C for 15-30 minutes, then temperature raised to 20°C and the reaction mass was stirred at 20°C for 4 hrs. The reaction mass was decomposed in water (1500 ml). The contents were stirred for 10-15 minutes followed by toluene was added to the reaction mass. The layers were separated, and the aqueous layer was extracted with toluene followed by separated the organic layer. Total organic layer was washed with water (1000 ml). The organic layer was then washed with brine solution (NaCl (50 gms) + water (500 ml)) and dried over sodium sulphate. The solvent was distilled out under vacuum. Petroleum ether (1000 ml) was added to the residue and the contents were cooled to below 10°C then stirred for 30 minutes. The solid was filtered off and washed with petroleum ether (200 ml). The solid was dried at 50-60°C for 3-5 hrs (Yield - 470 gms; 87.38%).
References:
AUROBINDO PHARMA LIMITED;CHANDIRAN, Takshinamoorthy;MEENAKSHISUNDERAM, Sivakumaran;SREENIVASA REDDY, Mundla WO2018/207120, 2018, A1 Location in patent:Page/Page column 11
446-36-6
268 suppliers
$9.00/10g

448-19-1
297 suppliers
$5.00/1g
456-49-5
361 suppliers
$8.00/10g

448-19-1
297 suppliers
$5.00/1g