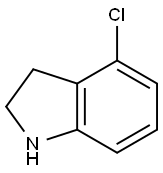
4-Chloroindoline synthesis
- Product Name:4-Chloroindoline
- CAS Number:41910-64-9
- Molecular formula:C8H8ClN
- Molecular Weight:153.61
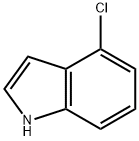
25235-85-2
323 suppliers
$6.00/1g

41910-64-9
116 suppliers
$40.00/1 g
Yield: 99%
Reaction Conditions:
Stage #1:4-chloro-1H-indole with sodium cyanoborohydride;acetic acid in N,N-dimethyl-formamide at 20; for 2 h;
Stage #2: with water;sodium hydroxide in N,N-dimethyl-formamide
Steps:
14 5.1.14. 4-Chloro-2,3-dihydro-1H-indole (15b)
General procedure: 5.1.24. 7-Fluoro-2,3-dihydro-1H-indole (15f) 7-Fluoro-1H-indole (20.0 g, 148 mmol) was dissolved in acetic acid (60 mL) and sodium cyanoborohydride (18.7 g, 296 mmol) was added in portions. The mixture was stirred for 2 h and then poured into 1500 mL of 2 M aqueous NaOH solution. The mixture was extracted with CH2Cl2. The organic layers were combined, washed with brine, dried over MgSO4, and concentrated under reduced pressure to give the title compound (20.0 g, 98%). 1H NMR (400 MHz, CDCl3) δ 3.08 (2H, t, J = 8.4 Hz), 3.62 (2H, t, J = 8.4 Hz), 6.62-6.66 (1H, m), 6.78-6.83 (1H, m), 6.90 (1H, dd, J = 7.6, 0.4 Hz).
References:
Sato, Kenjiro;Sugimoto, Hiromichi;Rikimaru, Kentaro;Imoto, Hiroshi;Kamaura, Masahiro;Negoro, Nobuyuki;Tsujihata, Yoshiyuki;Miyashita, Hirohisa;Odani, Tomoyuki;Murata, Toshiki [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 5,p. 1649 - 1666]
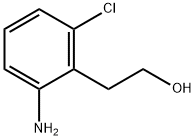
100376-53-2
20 suppliers
$45.00/10mg

41910-64-9
116 suppliers
$40.00/1 g
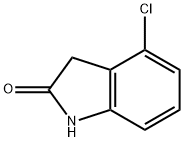
20870-77-3
118 suppliers
$7.00/100mg

41910-64-9
116 suppliers
$40.00/1 g

25235-85-2
323 suppliers
$6.00/1g
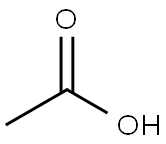
64-19-7
1560 suppliers
$10.00/25ML

41910-64-9
116 suppliers
$40.00/1 g
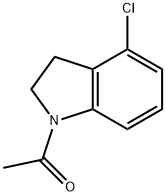
860024-86-8
3 suppliers
inquiry
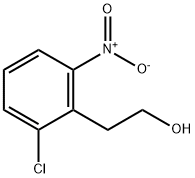
102493-68-5
15 suppliers
inquiry

41910-64-9
116 suppliers
$40.00/1 g