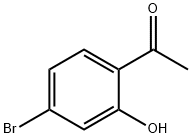
4-BROMO-2-HYDROXYACETOPHENONE synthesis
- Product Name:4-BROMO-2-HYDROXYACETOPHENONE
- CAS Number:30186-18-6
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
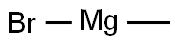
75-16-1
269 suppliers
$12.00/10ml

288067-35-6
167 suppliers
$5.00/250mg

30186-18-6
194 suppliers
$6.00/1g
Yield:30186-18-6 71.9%
Reaction Conditions:
Stage #1:methylmagnesium bromide;4-bromo-2-hydroxy-benzonitrile in tetrahydrofuran;toluene at 0 - 20; for 15 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water;toluene at 0; for 2 h;Heating / reflux;
Steps:
1.3
To a stirred solution of 4-Bromo-2-hydroxy-benzonitrile (3. 6 g, 18. 2MMOL) in anhydrous THF solution (20 mL) was added methylmagnesium bromide (1. 4 M in THF/toluene, 39.0 mL, 54. 6MMOL, 3 eq) at 0 °C under argon. The reaction mixture was slowly warm up to rt and stirred for 15 h. The mixture was cooled to 0 °C and quenched by the addition of water (5 mL) followed by concentrated HCI (10 mL). The resulting solution was refluxed for 2 h before cooled down to rt. The reaction mixture was then poured into EtOAc (100 mL) and water (100 mL). The layer was separated and the organic layer was washed with water (2 x 50 mL), dried over NA2SO4, filtrated, and concentrated under reduced pressure. The residue was then subjected to silica gel chromatography (20% EtOAc/Hexane) to provide 1- (4-BROMO-2-HYDROXY-PHENYL)- ethanone (2.81 g, 71.9%) as yellow OIL. 1H-NMR (CDCI3) : 8 12.32 (s, 1H, OH), 7.57 (d, J = 8. 7HZ, 1H), 7.17 (d, J = 1,9 Hz, 1H), 7.03 (dd, J = 1.9, 8.7 Hz, 1H), 2.62 (s, 3H).
References:
BAYER PHARMACEUTICALS CORPORATION WO2005/14566, 2005, A1 Location in patent:Page/Page column 61
35065-86-2
88 suppliers
$23.00/1g

30186-18-6
194 suppliers
$6.00/1g
591-20-8
346 suppliers
$10.00/1g
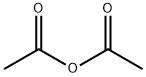
108-24-7
5 suppliers
$14.00/250ML

30186-18-6
194 suppliers
$6.00/1g
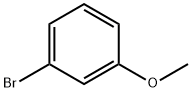
2398-37-0
579 suppliers
$8.00/10g
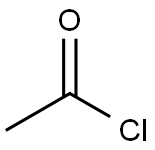
75-36-5
578 suppliers
$17.92/100G

30186-18-6
194 suppliers
$6.00/1g

591-20-8
346 suppliers
$10.00/1g

75-36-5
578 suppliers
$17.92/100G

30186-18-6
194 suppliers
$6.00/1g