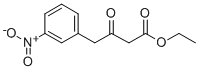
4-(3-NITRO-PHENYL)-3-OXO-BUTYRIC ACID ETHYL ESTER synthesis
- Product Name:4-(3-NITRO-PHENYL)-3-OXO-BUTYRIC ACID ETHYL ESTER
- CAS Number:116904-71-3
- Molecular formula:C12H13NO5
- Molecular Weight:251.24
Yield:-
Reaction Conditions:
with 1,1'-carbonyldiimidazole in tetrahydrofuran
Steps:
111.1 4-[3-[[[(3R,5S)-7-Chloro-5-(2,3-dimethoxyphenyl)-1-(3-hydroxy-2,2-dimethylpropyl)-2-oxo-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]acetyl]amino]phenyl]butanoic acid
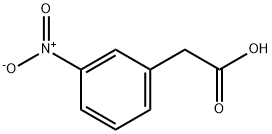
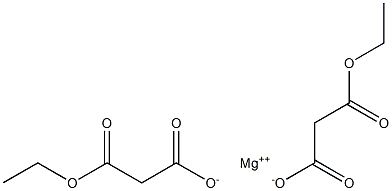
(1) To a solution of 3-nitrophenylacetic acid (10 g, 55.2 mmol) in tetrahydrofuran (100 ml) was added carbonyldiimidazole (10.5 g, 65.0 mmol). After stirring at room temperature for 6 hours, magnesium salt of monoethyl malonate (9.3 g, 32.5 mmol) were added thereto. The mixture was stirred at 60° C. for 3 hour. Then, the reaction mixture was diluted with ethyl acetate (100 ml) and washed with 1 N hydrochloric acid, saturated aqueous sodium hydrogen carbonate solution and saturated saline. After drying with sodium sulfate, the mixture was concentrated under reduced pressure. The residue was purified by silica gel column chromatography [eluent:hexane-ethyl acetate (1:1)] to obtain ethyl 4-(3-nitrophenyl)-3-oxobutanoate (10.0 g, 39.8 mmol, 72%) as a colorless amorphous powder. IR νmax (KBr) cm-1: 1745, 1722 (C=O). 1H-NMR (CDCl3) δ: 1.297 (3H, t, J=7.4 Hz), 3.544 ({fraction (9/10)}*2H, s), 3.606 ({fraction (1/10)}*2H, s), 4.005 ({fraction (9/10)}*2H, s), 4.195 ({fraction (1/10)}*2H, q, J=7.4 Hz), 4.223 ({fraction (9/10)}*2H, q, J=7.4 Hz), 4.982 ({fraction (1/10)}*1H, s), 7.52-7.55 (2H, m), 8.08-8.19 (2H, m).
References:
Kori, Masakuni;Miki, Takashi;Nishimoto, Tomoyuki;Tozawa, Ryuichi US2003/78251, 2003, A1

2033-24-1
658 suppliers
$6.00/25g

64-17-5
727 suppliers
$10.00/50g
38411-41-5
1 suppliers
inquiry

116904-71-3
6 suppliers
inquiry

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)