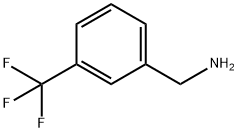
3-(Trifluoromethyl)benzylamine synthesis
- Product Name:3-(Trifluoromethyl)benzylamine
- CAS Number:2740-83-2
- Molecular formula:C8H8F3N
- Molecular Weight:175.15
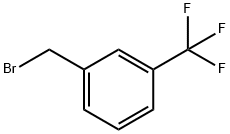
402-23-3
261 suppliers
$6.00/1g
![Pyrrolo[1,2-a]pyrimidine-6-carboxylic acid, 4,6,7,8-tetrahydro-4-oxo-3-[[(phenylmethoxy)carbonyl]amino]-, (6S)-](/CAS/20210111/GIF/227616-77-5.gif)
227616-77-5
1 suppliers
inquiry

2740-83-2
176 suppliers
$10.00/1g
![Pyrrolo[1,2-a]pyrimidine-6-carboxylic acid, 4,6,7,8-tetrahydro-4-oxo-3-[[(phenylmethoxy)carbonyl][[3-(trifluoromethyl)phenyl]methyl]amino]-, (6S)-](/CAS/20210305/GIF/437761-03-0.gif)
437761-03-0
0 suppliers
inquiry
Yield:2740-83-2 6.18g (93%)
Reaction Conditions:
with NaH;tetra-(n-butyl)ammonium iodide in tetrahydrofuran;water;
Steps:
12 (6S)-3-{[(Benzyloxy)carbonyl][3-(trifluoromethyl)benzyl]amino}-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxylic acid (12a)
(6S)-3-{[(Benzyloxy)carbonyl][3-(trifluoromethyl)benzyl]amino}-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxylic acid (12a) To a mixture of acid 1i (4.50 g, 13.7 mmol) in 70 mL THF at 0° C., was added 3-(trifluoromethyl)benzyl bromide (8.35 mL, 54.7 mmol), NaH (60% dispersion in oil, 1.64 g, 41.4 mmol), and TBAI (100 mg, catalytic). The reaction was stirred at rt for 15 h, then quenched with the addition of 50 mL H2O. The volatile solvents were removed by rotary evaporation and the aqueous solution obtained was partitioned with Et2O. The organic phase was extracted with 20% sat. NaHCO3 (3*). The combined organic extract was acidified with 1N HCl and extracted with EtOAc (5*). The combined organic extract was washed (brine), dried (Na2SO4), and concentrated to afford 6.18g (93%) of the 3-(trifluoromethyl)benzyl amine (12a), which was used in the following step without additional purification.
References:
US2003/64962,2003,A1
368-77-4
272 suppliers
$10.00/10g

2740-83-2
176 suppliers
$10.00/1g
454-89-7
385 suppliers
$14.00/25g

2740-83-2
176 suppliers
$10.00/1g
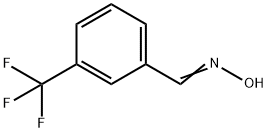
368-83-2
36 suppliers
$45.00/250mg

2740-83-2
176 suppliers
$10.00/1g