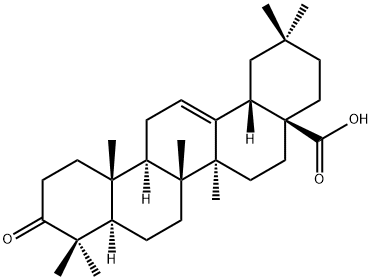
3-Oxo-olean-12-en-28-oic acid synthesis
- Product Name:3-Oxo-olean-12-en-28-oic acid
- CAS Number:17990-42-0
- Molecular formula:C30H46O3
- Molecular Weight:454.68
508-02-1
535 suppliers
$29.00/1g

17990-42-0
134 suppliers
inquiry
Yield:17990-42-0 99%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 20; for 1 h;Inert atmosphere;
Steps:
III.i (i) Preparation of Ib: (4aS,6aS,6bR,12aR)-2,2,6a,6b,9,9,12a-Heptamethyl-10-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid
(i)
Preparation of Ib: (4aS,6aS,6bR,12aR)-2,2,6a,6b,9,9,12a-Heptamethyl-10-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid
To a mixture of oleanolic acid (Ia, 5.0 g, 10.9 mmol) and CH2Cl2 (200 mL) was added the Dess-Martin reagent (6.0 g, 14.2 mmo l) under nitrogen at room temperature.
After stirring at room temperature for 1 hour, the starting material was consumed, as indicated by TLC (1:1 hexanes:diethyl ether).
The reaction mixture was quenched with the addition of a solution of sodium thiosulfate and NaHCO3 (50 g sodium thiosulfate in 200 mL saturated NaHCO3 solution).
The mixture was stirred at room temperature for 10 minutes.
The layers were separated and the aqueous layer was extracted with EtOAc (3 x 250 mL).
The combined organics were dried (Na2SO4), filtered and concentrated under reduced pressure.
The residue was purified by column chromatography (silica, 3:1 hexanes/diethyl ether) to provide the sub-title compound (4.9 g, 99%) as a white foam.
1H NMR (300 MHz, CDCl3) 0.81 (s, 3H), 0.90-2.05 (m, 39H), 2.42-2.44 (m, 1H), 2.59-2.61 (m, 1H), 2.83-2.85 (m, 1H), 5.29-5.31 (m, 1H). ESI MS m/z 455 [C30H46O3 + H]+.
References:
Sequoia Sciences, Inc.;Eldridge, Gary R.;Buckle, Ronald Neil;Ellis, Michael;Huang, Zhongping;Reilly, John Edward EP2712863, 2014, A1 Location in patent:Paragraph 0040
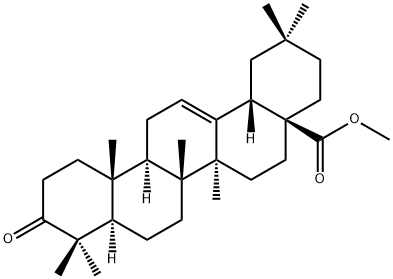
1721-58-0
24 suppliers
inquiry

17990-42-0
134 suppliers
inquiry
25499-90-5
60 suppliers
$85.00/5mg

17990-42-0
134 suppliers
inquiry

508-02-1
535 suppliers
$29.00/1g
1066-30-4
232 suppliers
$30.00/100g

17990-42-0
134 suppliers
inquiry