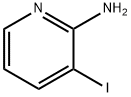
3-Iodopyridin-2-amine synthesis
- Product Name:3-Iodopyridin-2-amine
- CAS Number:104830-06-0
- Molecular formula:C5H5IN2
- Molecular Weight:220.01
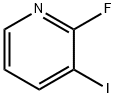
113975-22-7
304 suppliers
$7.00/1g

104830-06-0
232 suppliers
$7.00/250mg
Yield:104830-06-0 95%
Reaction Conditions:
with acetamidine hydrochloride;sodium hydroxide in water;dimethyl sulfoxide at 130; for 24 h;
Steps:
1 preparation of 3-iodo-2-aminopyridine
2-Fluoro-3-iodopyridine (1 mmol, 223.0 mg), acetamidine hydrochloride (1.2 mmol, 113.4 mg), NaOH (2.5 mmol, 100 mg), H2O (0.5 mL) and Dimethyl sulfoxide (2.5 mL). The reaction was carried out at 130 ° C for a reaction time of 24 hours. After the reaction was completed, it was cooled to room temperature.The reaction was quenched by adding 10 mL of ethyl acetate, and washed with 6 mL of saturated brine.The organic phase was separated, and the aqueous phase was extracted three times with ethyl acetate (6 mL of ethyl acetate each time). The organic phase was combined, dried over anhydrous sodium sulfate, and the solvent was removed by distillation under reduced pressure, including organic solvent and inorganic solvent. Organic solvent,The title product 3-iodopyridin-2-amine was isolated by column chromatography to give a yield of 95%.
References:
Wuyi University;Li Yibiao;Huang Guoling;Huang Shuo;Liao Chunshu;Zhong Zhengrong;Zhong Jingyi CN109232402, 2019, A Location in patent:Paragraph 0025; 0026
113975-31-8
81 suppliers
$24.00/1g

104830-06-0
232 suppliers
$7.00/250mg
13534-99-1
357 suppliers
$4.00/1g

104830-06-0
232 suppliers
$7.00/250mg
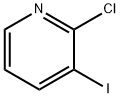
78607-36-0
361 suppliers
$14.00/1g

104830-06-0
232 suppliers
$7.00/250mg
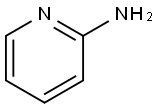
504-29-0
548 suppliers
$5.00/5 g

104830-06-0
232 suppliers
$7.00/250mg