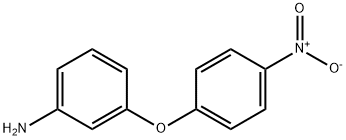
3-AMINO-4'-NITRODIPHENYL ETHER synthesis
- Product Name:3-AMINO-4'-NITRODIPHENYL ETHER
- CAS Number:22528-34-3
- Molecular formula:C12H10N2O3
- Molecular Weight:230.22
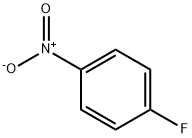
350-46-9
515 suppliers
$10.60/1gm:
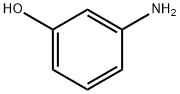
591-27-5
546 suppliers
$11.00/10g

22528-34-3
20 suppliers
inquiry
Yield:22528-34-3 95%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 80; for 8 h;
Steps:
A12.i
(i) Production of 3-(4-nitrophenoxy)aniline; To a solution of 1-fluoro-4-nitrobenzene (14.1 g, 100 mmol) and 3-aminophenol (11.2 g, 102 mmol) in N,N-dimethylformamide (150 mL) was added potassium carbonate (26.5 g, 191 mmol) and the mixture was stirred at 80°C for 8 hr. The reaction mixture was cooled to room temperature, diluted with ethyl acetate-hexane (1:1, 250 mL) and washed with water (250 mLx2) and saturated brine (100 mL), successively. The collected aqueous layer was extracted with ethyl acetate-hexane (1:1, 250 mL). The collected organic layer was dried over anhydrous magnesium sulfate, and decolorized with activated carbon. The insoluble material was filtered off through a pad filled with two layers of silica gel and celite, and the filtrate was concentrated. The obtained yellow syrup-like substance was recrystallized from ethyl acetate and hexane to give the title compound (21.8 g, 95%) as yellow crystals. 1H-NMR (DMSO-d6, 300 MHz) δ5.39 (2H, br s), 6.26 (1H, ddd, J = 0.9, 2.4, 7.8 Hz), 6.31 (1H, t, J = 2.1 Hz), 6.48 (1H, ddd, J = 0.9, 2.1, 8.1 Hz), 7.07-7.14 (3H, m), 8.24 (2H, dt, J = 7.2, 3.6 Hz).
References:
EP2181987,2010,A1 Location in patent:Page/Page column 77
76506-19-9
0 suppliers
inquiry

22528-34-3
20 suppliers
inquiry
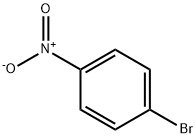
586-78-7
5 suppliers
$14.00/5g

22528-34-3
20 suppliers
inquiry
![Acetamide,N-[3-(4-nitrophenoxy)phenyl]-](/CAS/20180601/GIF/22483-34-7.gif)
22483-34-7
0 suppliers
inquiry

22528-34-3
20 suppliers
inquiry
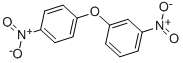
2914-72-9
13 suppliers
inquiry
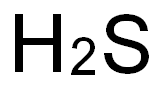
7783-06-4
74 suppliers
$158.00/25ml

7664-41-7
508 suppliers
$15.00/100ml

22528-34-3
20 suppliers
inquiry