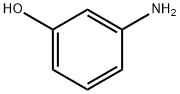
3-Aminophenol
- CAS No.
- 591-27-5
- Chemical Name:
- 3-Aminophenol
- Synonyms
- M-AMINOPHENOL;META AMINO PHENOL;m-Aminofenol;3-HYDROXYANILINE;1-Amino-3-hydroxybenzene;3-AMINO-1-HYDROXYBENZENE;nakoteg;renaleg;ursoleg;furroeg
- CBNumber:
- CB8853903
- Molecular Formula:
- C6H7NO
- Molecular Weight:
- 109.13
- MDL Number:
- MFCD00007786
- MOL File:
- 591-27-5.mol
- MSDS File:
- SDS
| Melting point | 120-124 °C (lit.) |
|---|---|
| Boiling point | 164 °C/11 mmHg (lit.) |
| Density | 0.99 |
| vapor pressure | 0.019Pa at 20℃ |
| refractive index | 1.5444 (estimate) |
| Flash point | 155 °C |
| storage temp. | 2-8°C |
| solubility | 26g/l |
| Colour Index | 76545 |
| form | Crystalline Powder |
| pka | 4.37, 9.82(at 20℃) |
| color | White to pinkish or light gray |
| Odor | odorless |
| PH | 6.8 (10g/l, H2O, 20℃) |
| Water Solubility | 35 g/L (20 ºC) |
| Merck | 14,460 |
| BRN | 636059 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, bases, mineral acids. May be light or air-sensitive. |
| LogP | 0.183 at 20℃ |
| CAS DataBase Reference | 591-27-5(CAS DataBase Reference) |
| EWG's Food Scores | 3-5 |
| FDA UNII | L3WTS6QT82 |
| NIST Chemistry Reference | Phenol, 3-amino-(591-27-5) |
| EPA Substance Registry System | 3-Aminophenol (591-27-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H332-H319-H411 | |||||||||
| Precautionary statements | P261-P264-P273-P301+P312-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 20/22-51/53 | |||||||||
| Safety Statements | 28-61-28A | |||||||||
| RIDADR | UN 2512 6.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | SJ4900000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29222900 | |||||||||
| Hazardous Substances Data | 591-27-5(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 i.p. in mice: 4.5 mg/20g (Koelzer, Giesen) | |||||||||
| NFPA 704 |
|
3-Aminophenol price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.00420 | 3-Aminophenol for synthesis | 591-27-5 | 5G | $42.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00420 | 3-Aminophenol for synthesis | 591-27-5 | 250g | $88.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00420 | 3-Aminophenol for synthesis | 591-27-5 | 1kg | $249 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00420 | 3-Aminophenol for synthesis | 591-27-5 | 50kg | $5200 | 2024-03-01 | Buy |
| Sigma-Aldrich | 100242 | 3-Aminophenol 98% | 591-27-5 | 5g | $36.4 | 2024-03-01 | Buy |
3-Aminophenol Chemical Properties,Uses,Production
Chemical Properties
o-Aminophenol appears as colorless needles or as white crystalline substance turning tan to brown on exposure to air.
Chemical Properties
Light grey crystal powder
Uses
Dye intermediate, manufacture of p-aminosalicylic acid.
Uses
m-Aminophenol (MAP) is an important starting material for dyes, including a variety of leuco (or latent) dyes used in imaging technology, optical bleaches and fluorescent agents, drugs, agricultural chemicals; and high-performance polymers. Although the synthesis of m-aminophenol(s) by the reaction of resorcinol with ammonia or (di)alkylarnines has been known for about 100 years,the preferred manufacturing process had been for many years a route involving the sulfonation of nitrobenzene to m-nitrobenzenesulfonic acid, the reduction of the nitro group to give m-aminobenzenesulfonic acid, followed by the caustic fusion of sodium m-aminobenzenesulfonate to yield sodium m-aminophenolate. The m-aminophenol was isolated/purified by neutralization, filtration, and recrystallization; the overall yield of m-aminophenol from nitrobenzene was estimated by SRI International to be 58%. (Doubtless, process improvements were made by the producers using this process.) The nitrobenzene/sulfonationlreductionlcaustic fusion route is still being used commercially, notably by ACNA in Italy, the largest producer of MAP, as well as a few smaller manufacturers. Sumitomo Chemical Co. used this process as well into the early 1980s and had licensed their knowhow to Hindustani Organic Chemicals, Ltd. of India which started up a plant on this basis with a capacity for MAP of 1.5 mill. lb /yr in 1970.The output of the Indian plant was said to be devoted to making sodium p-aminosalycilate (PAS), a tuberculostatic agent.
Uses
3-Aminophenol is an aromatic compound used in the preparation of inhibitors of mammalian carbonic anhydrase isoforms. Also used in the preparation of quinoline tethered fluorescent carbon dots for regulated anticancer discovery.
Application
3-Aminophenol has been used in the synthesis of disulfonated bis[4-(3-aminophenoxy)phenyl]sulfone (S-BAPS).
It can be used to synthesize:
Methyl 2-oxo-7-[(triphenylphosphoranylidene)amino]-2H-chromene-4-carboxylate by reacting with dimethyl acetylenedicarboxylate (DMAD) in the presence of triphenylphosphine.
3-Amino-2-cyclohexen-1-one via palladium-catalyzed hydrogenation.
Definition
ChEBI: An aminophenol that is one of three amino derivatives of phenol which has the single amino substituent located meta to the phenolic -OH group.
Synthesis Reference(s)
Tetrahedron Letters, 25, p. 1479, 1984 DOI: 10.1016/S0040-4039(01)80191-X
General Description
White crystals or off-white flakes.
Air & Water Reactions
3-Aminophenol may be sensitive to prolonged exposure to air and light. Slightly soluble in water.
Reactivity Profile
3-Aminophenol may react with strong oxidizers and mineral acids or bases.
Hazard
Toxic by ingestion.
Fire Hazard
Flash point data for 3-Aminophenol are not available. 3-Aminophenol is probably combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion, subcutaneous, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. A skin and eye irritant. When heated to decomposition it emits toxic fumes of NOx,.
Synthesis
3-Amino-2-cyclohexene-1-one (1.5 mol, 166.7 g), potassium acetate (6 mmol, 0.6 g), and 5% palladium on carbon (4.7 g, 65.5% water) were added to N, N-dimethylacetamide (475 g) solvent in one liter creased round bottom flask equipped with a nitrogen purge, thermometer, condenser, distillation head, overhead stirrer, and heating mantle. The solution was then heated to reflux for two hours at 173° C. with a continuous nitrogen purge. The palladium catalyst was filtered from the solution. Gas chromatography analysis indicated 130.9 g 3-aminophenol (1.2 mole, 80% yield based on 3-amino-2-cyclohexene-1-one).
Potential Exposure
Workers may be exposed to oAminophenol during its use as a chemical intermediate; in the manufacture of azo and sulfur dyes; and in the photographic industry. There is potential for consumer exposure to o-Aminophenol because of its use in dyeing hair, fur, and leather. The compound is a constituent of 75 registered cosmetic products suggesting the potential for widespread consumer exposure. p-Aminophenol is used mainly as a dye, dye intermediate and as a photographic developer; and in small quantities in analgesic drug preparation. Consumer exposure to p-aminophenol may occur from use as a hairdye or as a component in cosmetic preparations. mAminophenol is used mainly as a dye intermediate
Shipping
UN2512 Aminophenols (o-; m-; p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Crystallise it from hot water or toluene. [Beilstein 13 IV 952.]
Incompatibilities
These phenol/cresol materials can react with oxidizers; reaction may be violent. Incompatible with strong reducing substances such as alkali metals, hydrides, nitrides, and sulfides. Flammable gas (H2) may be generated, and the heat of the reaction may cause the gas to ignite and explode. Heat may be generated by the acidbase reaction with bases; such heating may initiate polymerization of the organic compound. Reacts with boranes, alkalies, aliphatic amines, amides, nitric acid, sulfuric acid. Phenols are sulfonated very readily (e.g., by concentrated sulfuric acid at room temperature). These reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid and can explode when heated. Many phenols form metal salts that may be detonated by mild shock.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
3-Aminophenol Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of5
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CD Chemical Group Limited | +8615986615575 | info@codchem.com | China | 20342 | 58 |
| HANGZHOU HAILAN CHEMICAL CO.,LTD. | +86-57156122185 +86-15967590394 | sales@hailan-chem.com | China | 400 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18751 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
View Lastest Price from 3-Aminophenol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-20 | 3-Aminophenol
591-27-5
|
US $0.00 / Kg/Bag | 1KG | 99% | 5000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-11-13 | 3-Aminophenol
591-27-5
|
US $10.00 / kg | 1kg | 99% | 100 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-10-17 | 3-Aminophenol
591-27-5
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Weibang Biotechnology Co., Ltd |
-

- 3-Aminophenol
591-27-5
- US $0.00 / Kg/Bag
- 99%
- Jinan Finer Chemical Co., Ltd
-

- 3-Aminophenol
591-27-5
- US $10.00 / kg
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- 3-Aminophenol
591-27-5
- US $0.00-0.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd