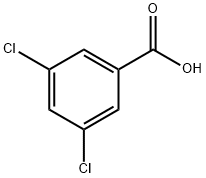
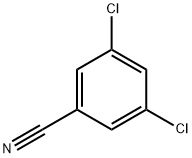
3,5-Dichlorobenzoic acid synthesis
- Product Name:3,5-Dichlorobenzoic acid
- CAS Number:51-36-5
- Molecular formula:C7H4Cl2O2
- Molecular Weight:191.01
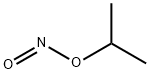
6575-00-4
260 suppliers
$6.00/1g

51-36-5
369 suppliers
$7.00/25g
Yield:51-36-5 93%
Reaction Conditions:
with water;sodium hydroxide for 6 h;Reflux;
Steps:
Synthesis of 3,5-Dichlorobenzoic Acid (2)
To a solution of sodium hydroxide (2.9 g, 0.073 mol) in 10mL of deionized water, 3,5-dichlorobenzonitrile (1) (5 g,0.029 mol) was added slowly. Under stirring, the mixturewas heated and refluxed for 6 h. The reaction was monitoredby TLC (methanol/ethyl acetate: v/v = 1/4). After thereaction completed, the mixture was cooled to room temperatureand the pH value of the solution was adjusted to1-3 by hydrogen chloride aqueous (2 mol/L). A white solidprecipitated, which was filtered under reduced pressure,washed with water, and dried to give a white powder. Thefiltrate was extracted with ethyl acetate. The white powderobtained in two crops was recrystallized from deionizedwater to obtain 3,5-dichlorobenzoic acid (2). Yield: 5.1 g(93%). FT-IR (KBr disc, cm-1): υ(C-H) 3084, υ(O-H) 2663,υ(C=O) 1706, υ(C-C) 1570 and 1448, β(C-O-H) 1403,β(C-H) 1289 and 1237, γ(C-H) 769. 1H NMR (400 MHz,DMSO-d6): δ(ppm) 7.90 (t, J = 2.0 Hz, 1H, Ph), 7.85 (d,J = 2.0 Hz, 2H, Ph).
References:
Zhang, Jun;Li, Yue;Wang, Bei;Jia, Ai-Quan;Zhang, Qian-Feng [Journal of Chemical Crystallography,2021,vol. 51,# 1,p. 108 - 115]
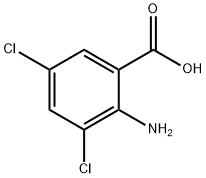
2789-92-6
207 suppliers
$18.00/1g

51-36-5
369 suppliers
$7.00/25g
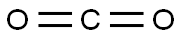
124-38-9
130 suppliers
$214.00/14L
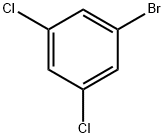
19752-55-7
358 suppliers
$8.00/10g

51-36-5
369 suppliers
$7.00/25g
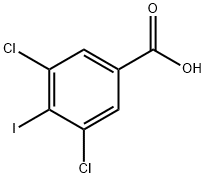
117757-68-3
18 suppliers
$45.00/2.5g
1765-93-1
560 suppliers
$9.00/1g

51-36-5
369 suppliers
$7.00/25g
![2,6-Dichloro-4'-fluoro-[1,1'-biphenyl]-4-carboxylic acid](/CAS/GIF/1350760-11-0.gif)
1350760-11-0
4 suppliers
inquiry