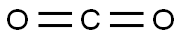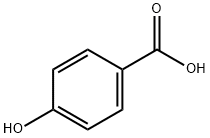
4-Hydroxybenzoic acid synthesis
- Product Name:4-Hydroxybenzoic acid
- CAS Number:99-96-7
- Molecular formula:C7H6O3
- Molecular Weight:138.12
In 1947, H. Gilman and C. E. Arntzen at Iowa State College (Ames) reported the synthesis of 4-HBA from 4-hydroxybenzaldehyde. Several other preparation methods were developed since then. Today it is produced commercially via the Kolbe–Schmitt reaction, which originated in the mid-1800s. Potassium phenoxide and CO2 are heated under pressure; the reaction mixture is then acidified to obtain the product. (Oddly, if the sodium salt is used, the product is 2-hydroxybenzoic acid, or salicylic acid.)
Org. Synth. 1934, 14, 48
DOI: 10.15227/orgsyn.014.0048
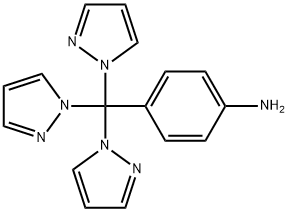
1000389-71-8
0 suppliers
inquiry

99-96-7
813 suppliers
$5.00/10g
Yield:99-96-7 99.3%
Reaction Conditions:
with sulfuric acid;sodium nitrite in water at 100; for 0.75 h;Cooling with ice;Concentration;
Steps:
2 Example 2
To a three-necked flask equipped with a reflux condenser, 3.05 g of 4-trispyrazolylmethylaniline was added,10 mL of water,2.5 ml of concentrated sulfuric acid was slowly added dropwise over 5 minutes,And 5.3 g of a 23.3% aqueous sodium nitrite solution was added thereto over 5 minutes on an ice bath,Stir for 15 min.A solution of 15.5% sulfuric acid in 1.1 mL was added dropwise over 5 minutes.The reaction was allowed to proceed for 15 minutes at 100 ° C,Natural cooling,The mixture was extracted three times with ethyl acetate,Each time 10mL,The organic phase was dried over anhydrous sodium sulfate,Ethyl acetate was removed by distillation under reduced pressure,Dried in vacuo to give a solid which was recrystallized from 19% dilute hydrochloric acid to give 1.37 g of white crystalline p-hydroxybenzoic acid in 99.3% yield).
References:
CN105481674,2016,A Location in patent:Paragraph 0021
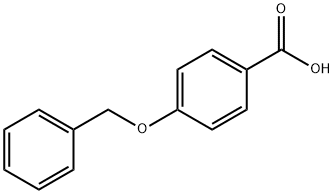
1486-51-7
214 suppliers
$6.00/1g

99-96-7
813 suppliers
$5.00/10g
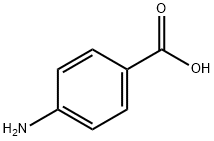
150-13-0
938 suppliers
$5.00/25g

99-96-7
813 suppliers
$5.00/10g
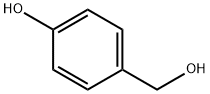
623-05-2
693 suppliers
$5.00/100mg

99-96-7
813 suppliers
$5.00/10g