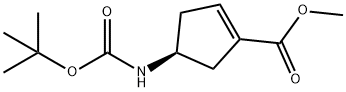
methyl (S)-4-((tert-butoxycarbonyl)amino)cyclopent-1-ene-1-carboxylate synthesis
- Product Name:methyl (S)-4-((tert-butoxycarbonyl)amino)cyclopent-1-ene-1-carboxylate
- CAS Number:251327-00-1
- Molecular formula:C12H19NO4
- Molecular Weight:241.28
![2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, 3-oxo-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/162427-15-8.gif)
162427-15-8
36 suppliers
inquiry

251327-00-1
17 suppliers
inquiry
Yield: 75%
Reaction Conditions:
with sodium methylate in methanol;dichloromethane
Steps:
9 (4S)-4-tert-butoxycarbonylaminocyclopent-1-enecarboxylic acid methyl ester
(4S)-4-tert-butoxycarbonylaminocyclopent-1-enecarboxylic acid methyl ester Sodium methoxide (8.23 ml of a 25% wt solution in methanol, 0.05 mol eq) was added dropwise to a stirred solution of (-)-N-Boc 2-azabicyclo[2.2.1]hept-5-en-3-one (150 g, 0.72 mol) in methanol (750 ml) at 0° C. After 30 minutes, the reaction was warmed to room temperature. After 60 h, the reaction was quenched with acetic acid and concentrated to dryness. The residue was redissolved in dichloromethane (700 ml) and washed with water (300 ml), saturated sodium hydrogen carbonate (300 ml) and brine (300 ml). The combined aqueous washings were then back extracted with dichloromethane (300 ml), the organic extracts combined and dried over magnesium sulfate. Filtration and concentration under reduced pressure gave a white solid which was recrystallized from ethyl acetate/heptane to give the title compound as white crystals (130 g, 75%) 1H-NMR (400 MHz, CDCl3); δ1.44 (9H, s, CH3 Boc), 2.32-2.49 (2H, m, 3-H), 2.85-3.01 (2H, m, 5-H), 3.74 (3H, s, CH3 ester), 4.36 (1H, app bs, 4-H), 4.74 (1H, app bs, NH), 6.69-6.75 (1H, m, 2-H).
References:
ChirotechTechnology Limited US6593489, 2003, B1
![4-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylic acid methyl ester](/CAS/GIF/168683-02-1.gif)
168683-02-1
164 suppliers
$161.00/10g

251327-00-1
17 suppliers
inquiry
![4-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylic acid methyl ester](/CAS/GIF/168683-02-1.gif)
168683-02-1
164 suppliers
$161.00/10g
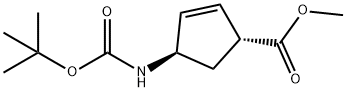
168958-19-8
25 suppliers
inquiry

251327-00-1
17 suppliers
inquiry
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
223 suppliers
$10.00/1g

251327-00-1
17 suppliers
inquiry
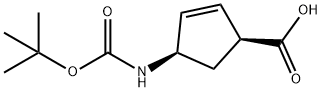
151907-79-8
167 suppliers
$20.00/100mg

251327-00-1
17 suppliers
inquiry