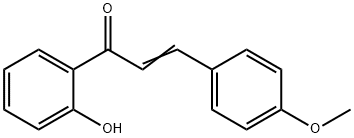
2'-HYDROXY-4-METHOXYCHALCONE synthesis
- Product Name:2'-HYDROXY-4-METHOXYCHALCONE
- CAS Number:3327-24-0
- Molecular formula:C16H14O3
- Molecular Weight:254.28
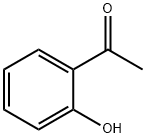
118-93-4
567 suppliers
$10.00/25g
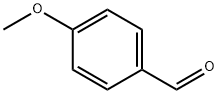
123-11-5
863 suppliers
$10.00/10g

3327-24-0
55 suppliers
$60.00/50mg
Yield:3327-24-0 100%
Reaction Conditions:
Stage #1: o-hydroxyacetophenonewith sodium hydroxide in ethanol;water at 0 - 5;
Stage #2: 4-methoxy-benzaldehyde in ethanol;water;
Steps:
2.1.1. General Procedure for the Synthesis of 3,4-dihydropyrimidine-2(1H)-thiones (1-13) [20]
General procedure: 2-Hydroxychalcones, the precursor of 3,4-DHPTMs, were prepared by the slow addition of 10% aq. sodium hy-droxide solution (2.0 mL) in the ethanolic solution of 2-hydroxyacetophenone (3.0 mmol, 0.40 ml) at 0-5oC. Then, (3.0 mmol, 320 mg) of benzaldehyde was slowly added to the reaction mixture on stirring. Upon the completion of re-action (monitored by TLC), the mixture was neutralized, and the precipitates formed were filtered, washed and recrystallized from ethanol. To proceed further, the chalcone (1.0 mmol) and thiourea (2.0 mmol) prepared in 10 mL of absolute ethanol were stirred for 10-15 minutes, and then alcoholic solution of KOH (0.002 mol, 10.0 ml) was added in portions. The obtained reaction mixture was refluxed further for 5-6 hours. After the completion of reaction (checked by TLC), the reaction mixture was allowed to cool at room temperature. The solvent was evaporated under reduced pressure and the crude product was purified by recrystalliza-tion in ethanol.
References:
Mughal, Ehsan Ullah;Sadiq, Amina;Hamayun, Muhammad;Zafar, Muhammad Naveed;Fatima, Nighat;Yameen, Muhammad Arfat;Muhammad, Syed Aun;Mumtaz, Amara;Ahmed, Ishtiaq;Fatima, Tehseen [Letters in drug design and discovery,2018,vol. 15,# 11,p. 1189 - 1201]

123-11-5
863 suppliers
$10.00/10g
582-24-1
246 suppliers
$8.00/5g

3327-24-0
55 suppliers
$60.00/50mg
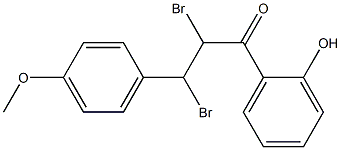
39729-17-4
0 suppliers
inquiry
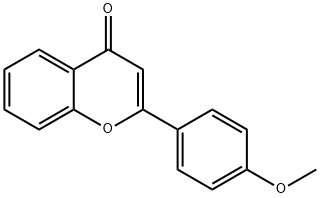
4143-74-2
70 suppliers
$45.00/2.5mg

3327-24-0
55 suppliers
$60.00/50mg
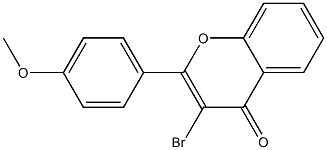
73910-85-7
1 suppliers
inquiry