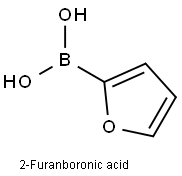
2-Furanboronic acid synthesis
- Product Name:2-Furanboronic acid
- CAS Number:13331-23-2
- Molecular formula:C4H5BO3
- Molecular Weight:111.89
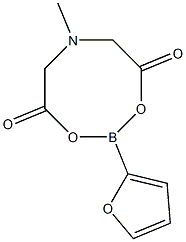
1104637-62-8
10 suppliers
$44.50/1g

13331-23-2
352 suppliers
$13.43/250mgs:
Yield:13331-23-2 95%
Reaction Conditions:
Stage #1:2-(furan-2-yl)-6-methyl-1,3,6,2-dioxazaborocane-4,8-dione with water;sodium hydroxide in tetrahydrofuran at 23; for 0.333333 h;
Stage #2: in tetrahydrofuran;diethyl ether; pH=7Aqueous phosphate buffer;
Steps:
5
The general method for synthesizing unprotected organoboronic acids was as follows. Under ambient atmosphere, to a 100 mL flask equipped with a stir bar and charged with MIDA boronate (2) (5 mmol) as a solution in THF (50 mL) was added aqueous NaOH (1.0 M, 15 mL). The mixture was vigorously stirred for 20 min. The mixture was then transferred to a separatory funnel and was diluted with EbO (50 mL) and 0.5 M pH 7 sodium phosphate buffer (50 mL). The mixture was shaken, and the phases were separated. The aqueous phase was extracted with THFiEt2O (1 :1 , 2 x 25 mL). The combined organics were dried over MgSO4, filtered and concentrated in vacuo. Residual solvent was co-evaporated with MeCN, and the resulting solid was placed under vacuum ( ~ 1 Torr) for 30 min. All boronic acids thus obtained were judged to be > 95% pure by 1 H-NMR and were utilized in cross-coupling reactions immediately after preparation. An example of this method is depicted in the scheme below. To form unprotected organoboronic acid 1a, the general procedure was followed using MIDA boronate 2a (1.127 g, 5.002 mmol) to yield the 1a as an off white solid (0.531 g, 95%). TLC (EtOAc) Rf = 0.46, stained with KMnO4. 1H-NMR (500 MHz, DMSO-d6:D2O 95:5 w/ TMS) δ 7.81 (dd, / = 1 .5, 0.5 Hz, 1 H), 7.07 (dd, / = 3.0, 0.5 Hz, 1 H), 6.48 (dd, / = 3.5, 2.0 Hz, 1 H). 13C-NMR (125 MHz, DMSO-d6:D2O 95:5 w/ TMS) δ 146.4, 121.5, 1 10.3. HRMS (El +) Calculated for C4HsO3B (M) + : 1 12.0332, Found: 1 12.0332.
References:
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS;BURKE, Martin, D.;KNAPP, David, M.;GILLIS, Eric, P. WO2010/36921, 2010, A2 Location in patent:Page/Page column 41
534-22-5
295 suppliers
$10.00/5mL
121-43-7
355 suppliers
$13.00/25mL

13331-23-2
352 suppliers
$13.43/250mgs:
110-00-9
209 suppliers
$10.00/25g
121-43-7
355 suppliers
$13.00/25mL
7732-18-5
470 suppliers
$12.69/100ml

13331-23-2
352 suppliers
$13.43/250mgs:
584-12-3
169 suppliers
$20.00/1g
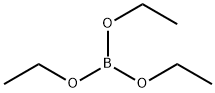
150-46-9
196 suppliers
$12.00/25mL

13331-23-2
352 suppliers
$13.43/250mgs:
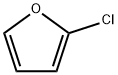
3187-94-8
7 suppliers
inquiry

13331-23-2
352 suppliers
$13.43/250mgs: