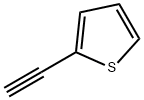
2-Ethynylthiophene synthesis
- Product Name:2-Ethynylthiophene
- CAS Number:4298-52-6
- Molecular formula:C6H4S
- Molecular Weight:108.16
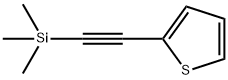
40231-03-6
93 suppliers
$45.00/1g

4298-52-6
157 suppliers
$24.00/1g
Yield:4298-52-6 92%
Reaction Conditions:
with potassium trimethylsilonate in dimethyl sulfoxide at 60; under 760.051 Torr; for 6 h;Catalytic behavior;Sealed tube;Reagent/catalyst;Solvent;
Steps:
33
2-[(trimethylsilyl)ethynyl]thiophene (1 mmol), inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilylate (sodium) (0.05 mmol), 2 mL of DMA or DMSO solvent was sequentially added to a 10 mL sealed tube and heated and stirred in a 60° C. oil bath for 6 hours. The progress of the reaction was followed by TLC. The reaction was completed and an equivalent of mesitylene or n-undecane was added to the crude product. The exact yield of the product was determined by GC and GC-MS. According to GC and GC-MS, when DMSO is used as a reaction solvent, inorganic base potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilylate (sodium) is used as a catalyst, and the yields of the products are as follows: 59%, 66%, 75%, 77%, 92%, 85%. When DMA was used as the reaction solvent, the inorganic potassium tert-butoxide (sodium) or potassium hydroxide (sodium) or potassium trimethylsilylate (sodium) was used as a catalyst, and the yield of the product was: 51%, 60%, respectively. 67%, 70%, 81%, 72%.
References:
Taizhou University;Yao Wubing CN107459438, 2017, A Location in patent:Paragraph 0080; 0081
77295-66-0
1 suppliers
inquiry

4298-52-6
157 suppliers
$24.00/1g
133844-84-5
6 suppliers
inquiry

4298-52-6
157 suppliers
$24.00/1g
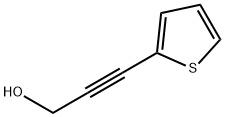
1194-13-4
5 suppliers
inquiry

4298-52-6
157 suppliers
$24.00/1g
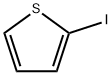
3437-95-4
242 suppliers
$6.00/5g
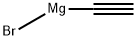
4301-14-8
124 suppliers
$55.20/100ml

4298-52-6
157 suppliers
$24.00/1g