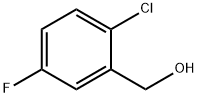
2-CHLORO-5-FLUOROBENZYL ALCOHOL synthesis
- Product Name:2-CHLORO-5-FLUOROBENZYL ALCOHOL
- CAS Number:261762-59-8
- Molecular formula:C7H6ClFO
- Molecular Weight:160.57
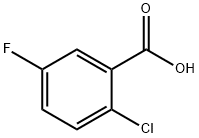
2252-50-8
245 suppliers
$5.00/1g

261762-59-8
89 suppliers
$9.00/1g
Yield:-
Reaction Conditions:
Stage #1:2-chloro-5-fluoro-benzoic acid with borane-THF in tetrahydrofuran at 0;Reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;water;ethyl acetateCooling with ice;
Steps:
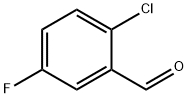
4.1.67. 2-Chloro-5-fluorobenzaldehyde
The solution of 2-chloro-5-fluoro-benzoic acid (4.00 g, 23.0 mmol) in THE (200 ml) was added triethylamine (4.14 ml, 29.9 mmol). At -30 °C, the mixture was added ethyl chloroformate (2.62 ml, 27.8 mmol) and stirred for 10 min. The solution was added sodium borohydrate (2.60 g, 68.9 mmol) and stirred for 5 min. The mixture was added H2O (10 ml) and stirred for 20 min at room temperature. The solution was concentrated in vacuo and the residue was added CHCl3 and 1 N HClaq. The organic layer was washed with 1 N NaOHaq and dried over Na2SO4. The solution was removed in reduced pressure. The residue was washed with n-hexane to give alcohol as a colorless solid. To the CH2Cl2 suspention of dimethylsulfoxide (2.43 ml, 34.2 mmol) was added a solution of oxalyl chloride (1.47 ml, 17.1 mmol) in 50 ml of dry CH2Cl2 at -78 °C, and the resulting mixture was stirred for 20 min. CH2Cl2 (15 ml) solution of the alcohol was added at -78 °C. The mixture was allowed to warm to -40 °C for 1 h. The mixture was added triethylamine (7.89 ml, 57.0 mmol), allowed to warm to room temperature, and was treated with 0.1 N HClaq. The organic layer was washed with saturated Na2CO3aq, dried over Na2SO4, and concentrated in vacuo to give the title compound (1.33 g, 37%) as a colorless solid after trituration with n-hexane. 1H NMR (400 MHz, CDCl3) δ 7.22-7.28 (1H, m), 7.41-7.47 (1H, m), 7.58-7.64 (1H, m), 10.42 (1H, d, J = 3.2 Hz). MS (EI) m/z 158 M+.
References:
Ichikawa, Masanori;Yokomizo, Aki;Itoh, Masao;Sugita, Kazuyuki;Usui, Hiroyuki;Shimizu, Hironari;Suzuki, Makoto;Terayama, Koji;Kanda, Akira [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 6,p. 1930 - 1949] Location in patent:experimental part
647020-63-1
45 suppliers
$30.00/1g

261762-59-8
89 suppliers
$9.00/1g
84194-30-9
180 suppliers
$14.00/1g

261762-59-8
89 suppliers
$9.00/1g