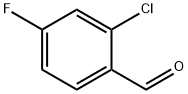
2-Chloro-4-fluorobenzaldehyde synthesis
- Product Name:2-Chloro-4-fluorobenzaldehyde
- CAS Number:84194-36-5
- Molecular formula:C7H4ClFO
- Molecular Weight:158.56
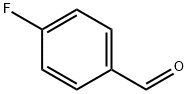
459-57-4
593 suppliers
$6.00/25g

84194-36-5
339 suppliers
$9.00/1g
Yield:84194-36-5 92%
Reaction Conditions:
with N-chloro-succinimide;sulfuric acid;trifluoroacetic acid at 0 - 70; for 23 h;Reagent/catalyst;Temperature;
Steps:
1-3 The preparation process of 2-chloro-4-fluorobenzaldehyde is as follows:
Add 250 ml of trifluoroacetic acid/concentrated sulfuric acid mixture into a 500 ml double-necked flask (the volume ratio of trifluoroacetic acid/concentrated sulfuric acid is 3/1), Under stirring, place in an ice-water bath, and add dropwise 24.8 g (100 mmol) of 4-fluorobenzaldehyde while maintaining the temperature at 0°C to 5°C; After the addition is complete, the temperature is raised to 70°C, and 3.34 g (25 mmol) of the first batch of N-chlorosuccinimide solid is added from the feeding port. After the addition, the bottle mouth is sealed and kept at 70°C with stirring Reaction for 4 hours; Open the feeding port, add the second batch of 3.34 grams (25 mmol) of N-chlorosuccinimide solid, and after sealing, keep stirring at 70°C for 4 hours; Open the feeding port, add 3.34 grams (25 mmol) of N-chlorosuccinimide solid in the third batch, and continue to stir and react at 70°C for 15 hours until the reaction is complete; Then it was cooled to room temperature, and the reaction solution was poured into 2 liters of ice water to quench the reaction. After phase separation, the aqueous phase and the organic phase were obtained; the aqueous phase was extracted with petroleum ether (100 mL×3), and the phase separation and the organic phase obtained by extraction were combined ; Then wash the organic phase with 25mL saturated sodium bicarbonate solution once, Wash the organic phase with 25 mL of saturated sodium chloride solution once, separate the liquids and dry the organic phase with anhydrous sodium sulfate, and finally concentrate under reduced pressure to remove the petroleum ether solvent. The crude product of 2-chloro-4-fluorobenzaldehyde was obtained. After the crude product was distilled under reduced pressure by an oil pump, 29.2 g of the pure product of 2-chloro-4-fluorobenzaldehyde was obtained. The pure product of the target product was colorless liquid. The yield was 92% by weight.
References:
Changzhou Technology College;Wu Zeying;Zhuang Yafeng;Cao Guiping;Ding Linlin;Zhang Zhenwei CN112079703, 2020, A Location in patent:Paragraph 0037-0063
167758-87-4
41 suppliers
$16.00/5g

84194-36-5
339 suppliers
$9.00/1g
112135-96-3
1 suppliers
inquiry

84194-36-5
339 suppliers
$9.00/1g
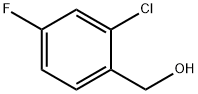
208186-84-9
125 suppliers
$17.00/5g

84194-36-5
339 suppliers
$9.00/1g
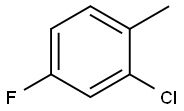
452-73-3
349 suppliers
$6.00/25g

84194-36-5
339 suppliers
$9.00/1g