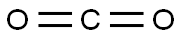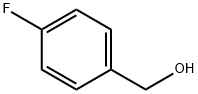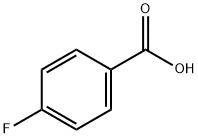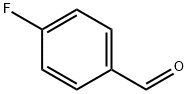
4-Fluorobenzaldehyde synthesis
- Product Name:4-Fluorobenzaldehyde
- CAS Number:459-57-4
- Molecular formula:C7H5FO
- Molecular Weight:124.11
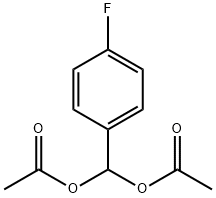
64002-52-4
0 suppliers
inquiry

459-57-4
593 suppliers
$6.00/25g
Yield:459-57-4 97%
Reaction Conditions:
with bovine liver acetone powder in aq. phosphate buffer;acetone at 20; pH=7.4; for 0.333333 h;Enzymatic reaction;chemoselective reaction;
Steps:
7 4.5. General deprotection protocol
General procedure: A solution of diacetate 2 (1mmol) and BLAP (10mg) in phosphate buffer (50mM, pH 7.4) was stirred in a vortex (200rpm) at 20°C for for an appropriate time as required to complete the reaction. After complete conversion, as indicated by GC or TLC, the reaction mixture was extracted with ethyl acetate (3×15mL). The combined organic layers were dried over anhydrous sodium sulfate and concentrated in vacuum to give the corresponding aldehyde 1.
References:
Koszelewski, Dominik;Ostaszewski, Ryszard [Bioorganic Chemistry,2019,vol. 93,art. no. 102825]
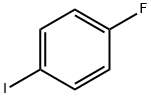
352-34-1
365 suppliers
$7.00/5g
201230-82-2
1 suppliers
inquiry
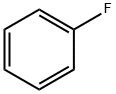
462-06-6
416 suppliers
$10.00/5g

459-57-4
593 suppliers
$6.00/25g
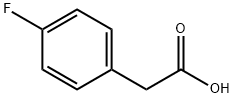
405-50-5
429 suppliers
$5.00/10g

459-57-4
593 suppliers
$6.00/25g