2,4-Dinitrofluorobenzene synthesis
- Product Name:2,4-Dinitrofluorobenzene
- CAS Number:70-34-8
- Molecular formula:C6H3FN2O4
- Molecular Weight:186.1
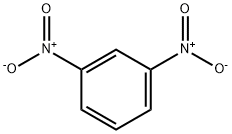
99-65-0
10 suppliers
$31.39/442244

70-34-8
6 suppliers
$10.00/5g
Yield:70-34-8 455 g
Reaction Conditions:
with fluorine in acetonitrile at 70; under 1500.15 Torr; for 1 h;Sealed tube;
Steps:
13
In the microreactor device system given in this article with two 27ml microreactors from Chemtrix (see also Figure 2), 104.5g/h (2.75mol/h) high concentration F2 gas ( Through Bronkhorst flowmeter) and 470.7g/h (2.8mol/h) 1,3-dinitrobenzene of CH3CN from the storage tank (tank) were fed together, the two microreactors were heated to 70, After the second microreactor, the pressure is adjusted to 2 bar absolute pressure through a pressure valve (the second microreactor in series only extends the residence time and has better temperature and pressure control for the reaction). The remaining material after the pressure valve is collected in a cooled stainless steel cylinder, followed by a scrubber, where the material from the microreactor enters the cylinder through a deep pipe. The post-treatment is performed by feeding the product mixture into ice water to remove HF. Analysis of the organic phase by GC-MS showed no starting material. The organic phase was rectified at 0.01 mbar and a condenser temperature of 30°C (after removing the solvent CH3CN in a rotary evaporator), 1-fluoro-2,4-dinitrobenzene was obtained at a transition temperature of 149°C. At 20°C, the distilled material becomes crystalline. In a reaction time of 1 hour, the isolated yield was about 89percent of the theoretical value (about 455 g).
References:
Fujian Yongjing Technology Co., Ltd.;Cui Weilong;Zhou Changyue;Du Hongjun;Wu Wenting CN111349018, 2020, A Location in patent:Paragraph 0326-0330
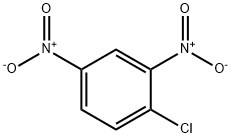
97-00-7
11 suppliers
$17.67/10gm:

70-34-8
6 suppliers
$10.00/5g
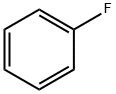
462-06-6
416 suppliers
$10.00/5g

70-34-8
6 suppliers
$10.00/5g
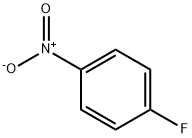
350-46-9
515 suppliers
$10.60/1gm:
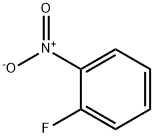
1493-27-2
512 suppliers
$5.00/10g