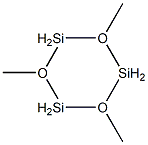
2 4 6-TRIMETHYLCYCLOTRISILOXANE 99 synthesis
- Product Name:2 4 6-TRIMETHYLCYCLOTRISILOXANE 99
- CAS Number:13269-39-1
- Molecular formula:C3H12O3Si3
- Molecular Weight:180.38
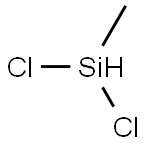
75-54-7
169 suppliers
$32.47/25G

13269-39-1
14 suppliers
inquiry
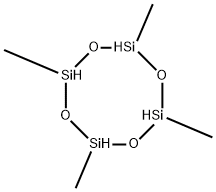
2370-88-9
258 suppliers
$17.00/5g
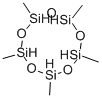
6166-86-5
47 suppliers
$95.00/2.5ml
Yield:-
Reaction Conditions:
with 18-crown-6 ether;water at 20; for 0.5 h;Overall yield = 95.4 %;
Steps:
5
General procedure: Hydrolysis of alkylchlorosilanes in a solution with a thermometer, a constant pressure dropping funnel and a reflux condenser, Gas) in a 500 ml three-necked flask. 95 g of methyldichlorosilane was uniformly dropped into a three-necked flask containing 95 g of water and 95 g of 6-crown-2 under magnetic stirring. The reaction temperature was controlled at below 20 °C, and the reaction was continued at the same temperature for 0.5 hour after the dropwise addition, and then the reaction solution was transferred to a separatory funnel to allow the solution to stand. The supernatant was subjected to vacuum distillation, get trimethylcyclotrisiloxane (D3H), tetramethylcyclotetrasiloxane (D4H), pentamethylcyclopentasiloxane (D5H), hexamethylcyclohexasiloxane (D6H). The contents of each component were analyzed by gas chromatograph. The results are shown in Table 1. The residual residue and the linear polysiloxane are regenerated under reduced pressure by acid or base, and then returned to the hydrolyzed oil to be re-rectified. The stratified layer of liquid is mainly crown ether and excess water. Can be used for hydrolysis of methyldichlorosilane continue to use. Such as lack of water, can add water. Such as strong acidity, alkali can be used after the use. The separated lower layer of liquid can also be cooled to a certain temperature, the crown ether precipitation, to achieve the purpose of recovery. The solvent in Example 1 was changed to 18-crown-6, i.e., 70 g of 16-crown-6 was dissolved in 95 g of water, according to the apparatus of Example 1.95 g of methyldichlorosilane was uniformly dropped into a three-necked flask under magnetic stirring and operated as in Example 1, and the results are shown in Table 1.
References:
CN104292253,2016,B Location in patent:Paragraph 0019; 0026; 0027; 0031

75-54-7
169 suppliers
$32.47/25G

13269-39-1
14 suppliers
inquiry

75-54-7
169 suppliers
$32.47/25G
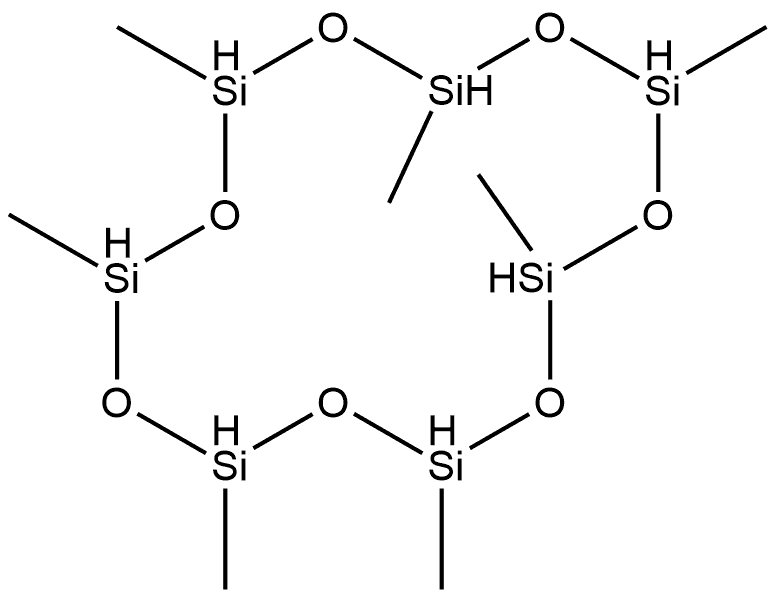
18291-55-9
0 suppliers
inquiry

13269-39-1
14 suppliers
inquiry

2370-88-9
258 suppliers
$17.00/5g
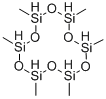
6166-87-6
4 suppliers
inquiry

75-54-7
169 suppliers
$32.47/25G

13269-39-1
14 suppliers
inquiry

2370-88-9
258 suppliers
$17.00/5g

6166-86-5
47 suppliers
$95.00/2.5ml

6166-87-6
4 suppliers
inquiry