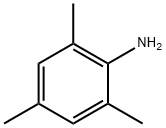
2,4,6-Trimethylaniline synthesis
- Product Name:2,4,6-Trimethylaniline
- CAS Number:88-05-1
- Molecular formula:C9H13N
- Molecular Weight:135.21
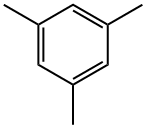
Mix sulfuric acid and nitric acid into mixed acid, nitrify with mesitylene at atmospheric pressure below 10°C for 4 hours, stand for stratification, and let off the waste acid. The obtained 2,4,6-trimethylnitrobenzene, iron powder, hydrochloric acid and water were prepared in a ratio of 1:3.74:0.9:2.22 (mol ratio) for reduction reaction at 100-105°C for 8h. Crude 2,4,6-Trimethylaniline was then distilled off from the reduced material and purified by distillation.
Yield:88-05-1 70%
Reaction Conditions:
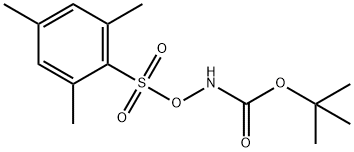
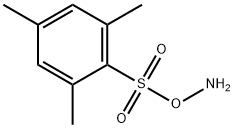
with Rh2(esp)2;trifluoroacetic acid in 2,2,2-trifluoroethanol at 0 - 20; for 25 h;Inert atmosphere;
Steps:
4 FIG. 2: Entry 4 (In Situ Generation Procedure)
To a 0° C. solution of 6 mesitylene (7a: 42 μL, 0.3 mmol) and 54 tert-butyl (mesitylsulfonyl)oxycarbamate (4d: 142 mg, 0.45 mmol, 1.5 equiv) in TFE (3 mL) was added 8 CF3CO2H (TFA: 46 μL, 0.6 mmol, 2 equiv) and 26 Rh2(esp)2 (4.55 mg, 6 μmol). The reaction was allowed to gradually warm to rt overnight. After 25 h, the reaction mixture (a suspension) was poured carefully into sat. aq. NaHCO3(15 mL) and extracted with EtOAc (3×7 mL). The combined organic extracts were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by PTLC (15% EtOAc/hexanes) to give 52 2,4,6-trimethylaniline (8c: 28.5 mg, 70%) as an oil whose spectral characteristics were concordant with literature values (see above).
References:
US2019/152892,2019,A1 Location in patent:Paragraph 0132; 0148; 0149
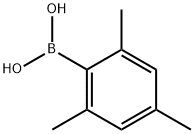
5980-97-2
261 suppliers
$10.00/1g

88-05-1
319 suppliers
$6.00/1g

603-71-4
80 suppliers
$328.00/500g

88-05-1
319 suppliers
$6.00/1g
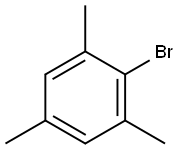
576-83-0
251 suppliers
$27.00/25G

88-05-1
319 suppliers
$6.00/1g