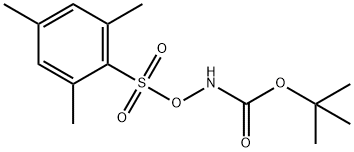
tert-Butyl (Mesitylsulfonyl)oxycarbaMate synthesis
- Product Name:tert-Butyl (Mesitylsulfonyl)oxycarbaMate
- CAS Number:36016-39-4
- Molecular formula:C14H21NO5S
- Molecular Weight:315.39
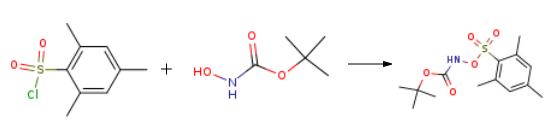
2-Mesitylenesufonylchloride (2.00 g, 9.17 mmol) was added to a flame dried and argon purged round-bottom flask and dissolved in ether (18 mL), followed by the addition of N-Boc-hydroxylamine (1.47 g, 1100 mmol) The flask was cooled to 0° C and then TEA was added dropwise (1.3 mL). The reaction stirred for 2 hours at 0° C, at which all starting material had been converted as seen by TLC. The TEA-Cl, white solid, was filtered, and washed with ether. The filtrate was concentrated in vacuo, and purified via flash chromatography in 25/75 EtOAc/hexane gradient. Yield was quantitative, 2.16 g, white solid.
773-64-8
266 suppliers
$5.00/5g
36016-38-3
355 suppliers
$6.00/5g

36016-39-4
97 suppliers
$19.00/250mg
Yield:36016-39-4 100%
Reaction Conditions:
with triethylamine in diethyl ether at 0; for 2 h;
Steps:
1 Preparation of N-Boc-O-(mesitylsulfonyl)hydroxylamine (N-Boc-MSH)
2-Mesitylenesufonylchloride (2.00 g, 9.17 mmol) was added to a flame dried and argon purged round-bottom flask and dissolved in ether (18 mL), followed by the addition of N-Boc-hydroxylamine (1.47 g, 1100 mmol) The flask was cooled to 0° C and then TEA was added dropwise (1.3 mL). The reaction stirred for 2 hours at 0° C, at which all starting material had been converted as seen by TLC. The TEA-Cl, white solid, was filtered, and washed with ether. The filtrate was concentrated in vacuo, and purified via flash chromatography in 25/75 EtOAc/hexane gradient. Yield was quantitative, 2.16 g, white solid. 1H NMR: (400 MHz, Chloroform-d) δ 7.77 (1H, s), 6.98 (2H, s), 2.66 (6H, s), 2.31 (3H, s), 1.30 (9H, s). 13C NMR: (100 MHz, CDCl3) δ 154.5, 144.7, 142.2, 131.9, 128.7, 84.1, 27.9, 23.4, 21.4. m/z: 315.0 [M+H].
References:
WO2019/157065,2019,A1 Location in patent:Paragraph 00152

773-64-8
266 suppliers
$5.00/5g
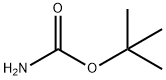
4248-19-5
377 suppliers
$5.00/5g

36016-39-4
97 suppliers
$19.00/250mg
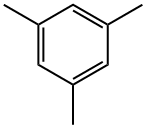
108-67-8
376 suppliers
$5.00/5G

36016-39-4
97 suppliers
$19.00/250mg