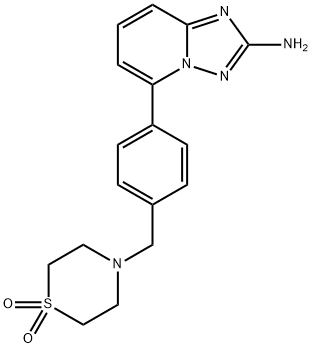
[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]- synthesis
- Product Name:[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]-
- CAS Number:1257705-09-1
- Molecular formula:C17H19N5O2S
- Molecular Weight:357.43
![Boronic acid, [4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]- (9CI)](/CAS/20200401/GIF/747413-23-6.gif)
747413-23-6
4 suppliers
inquiry
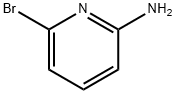
![5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine](/CAS2/GIF/1010120-55-4.gif)
1010120-55-4
163 suppliers
$6.00/100mg
![[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]-](/CAS/20200611/GIF/1257705-09-1.gif)
1257705-09-1
27 suppliers
inquiry
Yield:1257705-09-1 79%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;water;N,N-dimethyl-formamide at 80; for 18 h;Product distribution / selectivity;Inert atmosphere;Sealed tube;
Steps:
95.95a
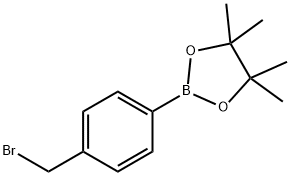
5- {4-[(l , 1 -dioxidothiomorpholin-4-yl)methyl]phenyl} [ 1 ,2,4]triazolo[ 1 ,5-a]pyridin- 2-amine was prepared from 5-bromo-[l,2,4]triazolo[l,5-a]pyridin-2-ylamine (368 mg, 1.73 mmol) and 4-[(4-boronophenyl)methyl]-thiomorpholine 1,1-dioxide (0.512 mg, 1.90 mmol), in a manner analogous to Step 73c. The residue was purified on a 12 g Isco silica gel column using a gradient of 0-15% methanol in dichloromethane as an eluent. 5- {4- [(1,1 -dioxidothiomorpholin-4-yl)methyl]phenyl} [ 1 ,2,4]triazolo[ 1 ,5-a]pyridin-2-amine (490 mg, 79 %) was isolated as a beige foam. 1H NMR (400 MHz, (D3C)2SO, δ, ppm): 7.93 (d, J = 8.5 Hz, 2H), 7.50 (m, 3H), 7.37 (d, J = 7.8 Hz, IH), 7.07 (d, J= 7.8 Hz, IH), 6.02 (s, 2H), 3.78 (s, 2H), 3.15 (s, 4H), 2.91 (s, 4H). MS = 358 (MH)+.
References:
WO2010/141796,2010,A2 Location in patent:Page/Page column 197; 198
![4-[4-(4,4,5,5-tetramethyl[1,3,2]dioxaborolan-2-yl)benzyl]thiomorpholine 1,1-dioxide](/CAS/20180629/GIF/1092563-25-1.gif)
1092563-25-1
68 suppliers
$65.00/10mg
![5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine](/CAS2/GIF/1010120-55-4.gif)
1010120-55-4
163 suppliers
$6.00/100mg
![[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]-](/CAS/20200611/GIF/1257705-09-1.gif)
1257705-09-1
27 suppliers
inquiry
1206161-97-8
226 suppliers
$25.00/1mg
![[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]-](/CAS/20200611/GIF/1257705-09-1.gif)
1257705-09-1
27 suppliers
inquiry
138500-85-3
262 suppliers
$8.00/1g
![[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]-](/CAS/20200611/GIF/1257705-09-1.gif)
1257705-09-1
27 suppliers
inquiry
19798-81-3
437 suppliers
$5.00/1g
![[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]-](/CAS/20200611/GIF/1257705-09-1.gif)
1257705-09-1
27 suppliers
inquiry