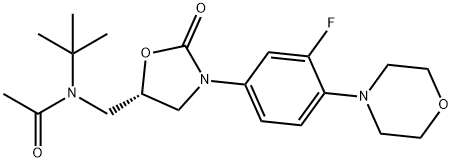
N-Tert-butyl-N-(((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide synthesis
- Product Name:N-Tert-butyl-N-(((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide
- CAS Number:1215006-11-3
- Molecular formula:C20H28FN3O4
- Molecular Weight:393.45
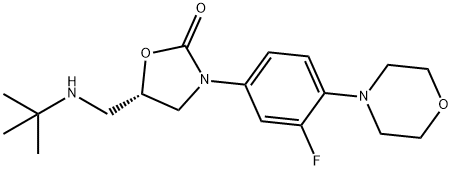
1215006-08-8
23 suppliers
inquiry
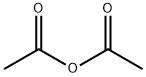
108-24-7
5 suppliers
$14.00/250ML

1215006-11-3
24 suppliers
inquiry
Yield:1215006-11-3 84%
Reaction Conditions:
with triethylamine in ethyl acetate at 55; for 18 h;Product distribution / selectivity;Inert atmosphere;
Steps:
7
Example 7 Preparation of N-tert-butyl-N-(((S)-3-(3-fluoro-4-morpholinoiphenyl)-2-oxazolidin-5-yl)methyl)acetamide (compound (II) with R1 = tert-butyl) To a suspension of (S)-5-((tert-butylamino)methyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one (1 g, 2.84 mmol) in ethyl acetate (2 mL, 2 vol) was added triethylamine (0.47 mL, 3.4 mmol, 1.2 eq) and acetic anhydride (0.32 mL, 3.4 mmol, 1.2 eq) and the mixture was heated to 55 °C for 18 h. The mixture was cooled to room temperature, was stirred at this temperature for 1 h and then at 0 °C for 1 h. Filtration, washing with cold ethyl acetate (2 x 1 mL, 1 vol) and drying in vacuo gave N-tert-butyl-N-(((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxazolidin-5-yl)methyl)acetamide (0.94 g, 84%, HPLC 99.2%, 99.9% ee) as a beige solid. 1H NMR (400 MHz, CDCl3): δ 7.44 (dd, 1H, ArH, J 2.8 and 14 Hz), 7.11 (ddd, 1H, ArH, J 1.2, 1, 2.4 and 9 Hz), 6.93 (t, 1H, ArH, J 9Hz), 4.78-4.72 (m, 1H, CH), 4.09 (t, 1H, CH, J 9 Hz), 3.88-3.86 (m, 5H, CHH + CH2), 3.74-3.63 (m, 3H, CH, CH2), 3.07-3.04 (m, 4H, CH2), 2.23 (s, 3H, CH3), 1.51 (s, 9H, CH3) ppm. Mp 185.5 - 186.9 °C [α]D -54.2 ° (c 1.03, CHCl3)
References:
EP2163547,2010,A1 Location in patent:Page/Page column 9

1215006-08-8
23 suppliers
inquiry
75-36-5
578 suppliers
$17.92/100G

1215006-11-3
24 suppliers
inquiry