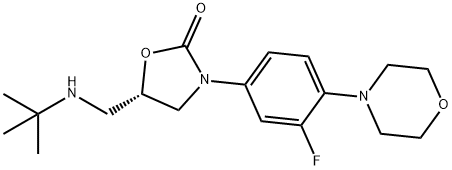
(S)-5-((Tert-butylamino)methyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one synthesis
- Product Name:(S)-5-((Tert-butylamino)methyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one
- CAS Number:1215006-08-8
- Molecular formula:C18H26FN3O3
- Molecular Weight:351.42
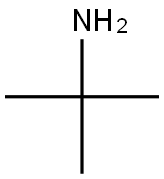
75-64-9
429 suppliers
$10.00/10g
![(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate](/CAS2/GIF/174649-09-3.gif)
174649-09-3
157 suppliers
$39.00/25mg

1215006-08-8
23 suppliers
inquiry
Yield:1215006-08-8 89%
Reaction Conditions:
in isopropyl alcohol at 120; for 72 h;Inert atmosphere;
Steps:
3
Example 3 Preparation of (S)-5-((tert-butylamino)methyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one (compound (IV) with R1 = tert-butyl) To a suspension of ((R)-3-(3-fluoro-4-morpholinophenyl)-2-oxazolidin-5-yl)methyl methanesulfonate (19 g, 50.7 mmol) in isopropanol (47.5 mL, 2.5 vol) in a sealed reactor was added tert-butylamine (13.3 mL, 126 mmol, 2.5 eq) and the mixture was heated to Tint 120 °C. After 3 d, the reaction is cooled to room temperature whereupon precipitation is observed. The suspension is then stirred for 1 h in an ice bath. Filtration, washing with isopropanol (2 x 19 mL, 1 vol) and water (2 x 19 mL, 1 vol) and drying gave (S)-5-((tertbutylamino)methyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one (15.3 g, 89%, HPLC 97.1%, 99.9% ee) as a white powder. 1H NMR (400 MHz, CDCl3): δ 7.45 (dd, 1H, ArH, J 2.8 and 14 Hz), 7.14 (dd, 1H, ArH, J 2 and 8 Hz), 6.93 (t, 1H, ArH, J 9Hz), 4.74-4.60 (m, 1H, CH), 3.99 (t, 1H, CH, J 9 Hz), 3.88-3.85 (m, 5H, CH2), 3.06-3.04 (m, 4H, CH2), 2.88 (qd, 2H, CH2, J 5, 6, 9 and 12 Hz), 1.1 (s, 9H, CH3) ppm. Mp 142.2 - 143.3 °C [α]D-52.4 (c 1.01, CHCl3)
References:
EP2163547,2010,A1 Location in patent:Page/Page column 8