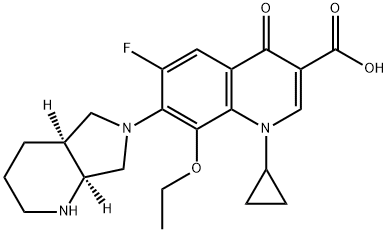
1-Cyclopropyl-8-ethoxy-6-fluoro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid synthesis
- Product Name:1-Cyclopropyl-8-ethoxy-6-fluoro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
- CAS Number:1029364-75-7
- Molecular formula:C22H26FN3O4
- Molecular Weight:415.46
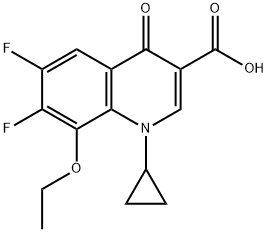
143158-55-8
41 suppliers
inquiry
![CIS-OCTAHYDROPYRROLO[3,4-B]PYRIDINE](/CAS/GIF/151213-40-0.gif)
151213-40-0
289 suppliers
$23.00/250mg
![1-Cyclopropyl-8-ethoxy-6-fluoro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid](/CAS/GIF/1029364-75-7.gif)
1029364-75-7
81 suppliers
$499.83/5MG
Yield:1029364-75-7 86.1%
Reaction Conditions:
Stage #1: C15H13F2NO4with isobutylamine;copper dichloride in methanol;ethylene glycol at 50; for 4 h;Inert atmosphere;
Stage #2: (1S,6S)-2,8-diazabicyclo[4.3.0]nonane in methanol;ethylene glycol; for 5 h;Reflux;
Steps:
5
The specific process is: adding 10 mL of methanol to a 25 mL reaction flask.Further compound 2-3 (0.99 g, 3.2 mmol), isobutylamine (0.42 g), copper dichloride (0.2 g),Ethylene glycol (0.22g), nitrogen protection, stirring at 50 ° C for 4 hours, then add 0.4g of compound 1 ((S,S)-2,8-diaza-bicyclo[4.3.0] decane), heated to reflux 5 After hours, filter,The filtrate was added to 4 mL of aqueous sodium hydroxide solution (2M), and the mixture was heated to reflux for 1 hour.The temperature was lowered to room temperature, and the pH of the reaction mixture was adjusted to 7-8 with 4M hydrochloric acid.The aqueous layer was extracted three times with 100 mL of ethyl acetate.Combine the organic layers, dry,Concentrated silica gel column chromatographyGotMoxifloxacin impurity C (1.14g),The yield was 86.1%.
References:
CN110183445,2019,A Location in patent:Paragraph 0055-0058
151096-09-2
306 suppliers
$13.00/100mg
![1-Cyclopropyl-8-ethoxy-6-fluoro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid](/CAS/GIF/1029364-75-7.gif)
1029364-75-7
81 suppliers
$499.83/5MG
![1-Cyclopropyl-6-fluoro-1,4-dihydro-8-hydroxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid](/CAS/GIF/721970-36-1.gif)
721970-36-1
82 suppliers
$498.63/5MG
![1-Cyclopropyl-8-ethoxy-6-fluoro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid](/CAS/GIF/1029364-75-7.gif)
1029364-75-7
81 suppliers
$499.83/5MG