Moxifloxacin synthesis
- Product Name:Moxifloxacin
- CAS Number:151096-09-2
- Molecular formula:C21H24FN3O4
- Molecular Weight:401.43
112811-72-0
319 suppliers
$9.00/5g
![CIS-OCTAHYDROPYRROLO[3,4-B]PYRIDINE](/CAS/GIF/151213-40-0.gif)
151213-40-0
289 suppliers
$23.00/250mg

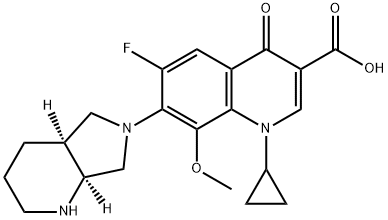
151096-09-2
306 suppliers
$13.00/100mg
Yield:151096-09-2 96%
Reaction Conditions:
with [BCl2(4-picoline)][AlCl4];triethylamine in methanol at 85; for 6 h;Catalytic behavior;Reagent/catalyst;Solvent;Temperature;
Steps:
1-5 Example 5
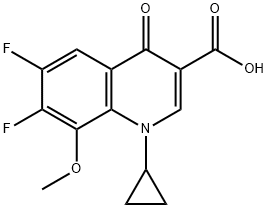
Add 1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (60g, 0.2mol) into a 500mL three-necked flask,Add 300mL methanol, and then add (S,S)-2,8-diazabicyclo[4.3.0]nonane (50.4g, 0.4mol),14mL of triethylamine and 1.2g of [BCl2(4pic)][AlCl4], stir mechanically until it is clear.After the addition is complete, the reaction solution is heated to 85°C for reaction.After TLC monitoring until the consumption of 1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid is complete, the reaction time is 6 hour,The reaction solution was cooled to room temperature, most of the solvent was evaporated under reduced pressure, the temperature of the system was slowly cooled to about 10°C, and the moxifloxacin monomer was precipitated.Filter, wash twice with methanol at the dosage of 10ml each time, wash twice with deionized water at the dosage of 15ml each time, and dry in vacuum.Moxifloxacin powdery solid 77g was obtained, the yield was 96%, and the purity was 99.8% as measured by HPLC area normalization method.1-cyclopropyl-6-{(S,S)-2,8-diazo-bicyclo[4.3.0]non-8-yl}-7-fluoro-8-methoxy-1,4-Dihydro-4-oxo-3-quinolinecarboxylic acid (C6-F substituted by-product) was not detected
References:
CN112759590,2021,A Location in patent:Paragraph 0029-0038
186826-86-8
711 suppliers
$5.00/50mg

151096-09-2
306 suppliers
$13.00/100mg
![3-Quinolinecarboxamide, 1-cyclopropyl-N,N-diethyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-](/CAS/20210305/GIF/1028205-69-7.gif)
1028205-69-7
0 suppliers
inquiry

151096-09-2
306 suppliers
$13.00/100mg
![1H-Pyrrolo[3,4-b]pyridine-1-carboxylic acid, 6-[1-cyclopropyl-3-[(diethylamino)carbonyl]-6-fluoro-1,4-dihydro-8-methoxy-4-oxo-7-quinolinyl]octahydro-, phenyl ester, (4aS,7aS)-](/CAS/20210305/GIF/1028205-70-0.gif)
1028205-70-0
0 suppliers
inquiry

151096-09-2
306 suppliers
$13.00/100mg
![1H-Pyrrolo[3,4-b]pyridine-1-carboxylic acid, 6-[1-cyclopropyl-3-[(diethylamino)carbonyl]-6-fluoro-1,4-dihydro-8-methoxy-4-oxo-7-quinolinyl]octahydro-, 1,1-dimethylethyl ester, (4aS,7aS)-](/CAS/20200611/GIF/1028205-71-1.gif)
1028205-71-1
0 suppliers
inquiry

151096-09-2
306 suppliers
$13.00/100mg