
Topotecan hydrochloride NEW
| Price | Get Latest Price |
| Package | 25kg |
| Min. Order: | 1kg |
| Supply Ability: | 2000ton |
| Update Time: | 2024-04-12 |
Product Details
| Product Name: Topotecan hydrochloride | CAS No.: 119413-54-6 |
| EC-No.: 601-607-9 | Min. Order: 1kg |
| Purity: 99% | Supply Ability: 2000ton |
| Release date: 2024/04/12 | |
| Appearance: White Powder |
Topotecan hydrochloride is a water-soluble camptothecin derivative, which is the hydrochloride form of Topotecan. It was successfully developed by SmithKlineBeecham Company in the United States and approved by FDA in 1996 under the product name Hycamtin for the treatment of ovarian cancer (OVC) as a second-line therapeutic drug. In 1999, the United States Food and Drug Administration (FDA) approved Topotecan hydrochloride as a treatment for small cell lung cancer (SCLC), which has been listed in dozens of countries and regions such as the United Kingdom, Germany and France. Phase III clinical trials for the treatment of non-small cell lung cancer, cervical cancer and myelodysplastic syndrome are ongoing. Topotecan hydrochloride can enter the blood-brain barrier, is orally as effective as intravenous administration, has low predictable myelosuppressive toxicity, and other non-hematological toxicity is less severe. At present, it has been produced by domestic manufacturers for the clinical treatment of small cell lung cancer, ovarian cancer and other tumors. Topotecan hydrochloride inhibits the activity of topoisomerase I, which is required for DNA replication. After intravenous injection, this product is hydrolyzed in the blood and excreted in urine. This product has a rapid serum clearance rate of 62L per hour, widely distributed in the body, and a half-life of about 2 to 3h. Preclinical tests showed that this product has antitumor activity against all kinds of tumor species. In Phase I clinical trials, this product also has significant antitumor effects on cisplatin resistant ovarian cancer patients.
Adverse reaction
In the limited dose of this product can appear bone marrow suppression and other toxic reactions, especially can cause neutropenia. It also often causes thrombocytopenia and anemia, sometimes requiring transfusions of red blood cells and platelets. Nausea, vomiting, hair loss, diarrhea, abdominal pain, gastritis and weakness may also occur, but they are mild.
Application:
1 Used as an antitumor agent
2 A topoisomerase I inhibitor.
Storage Condition | Keep in a cool and dry place |
Transportation | By Sea or by Air(DHL/UPS/TNT/FEDEX/EMS) |
Delivery Time | 7-28 days |
Payment | T/T, Western Union or Bitcoin |





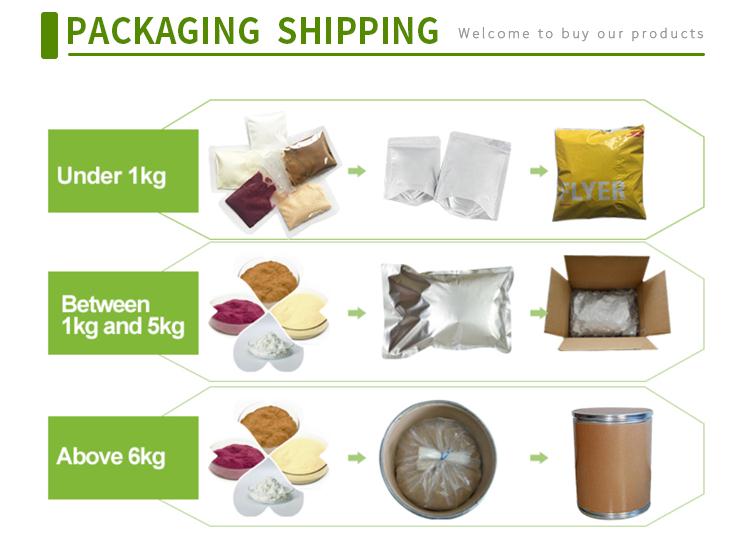




Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $30.00/5mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2025-04-28 | |
| $30.00/5mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2025-04-27 | |
| $10.00/1KG |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-11-28 | |
| $0.00/25KG |
VIP5Y
|
Hebei Mujin Biotechnology Co.,Ltd
|
2023-08-29 | |
| $0.00/1kg |
VIP2Y
|
Hangzhou ICH Biofarm Co., Ltd
|
2023-06-26 | |
| $100.00/1KG |
Hubei Harvest Chemical CO.,Ltd
|
2022-10-25 | ||
| $10.00/1kg |
Hebei Linwo New Material Technology Co., LTD
|
2022-07-08 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $1.10/1g |
VIP5Y
|
Dideu Industries Group Limited
|
2021-07-02 | |
| $0.00/1g |
VIP4Y
|
WUHAN FORTUNA CHEMICAL CO., LTD
|
2021-06-23 |
- Since: 2020-08-05
- Address: 804, Unit 2, Building 4, i City, No. 11, South Tangyan Road, High-tech Zone, Xi 'an, Shaanxi











 China
China