- Ambrisentan
-

- $0.00 / 1KG
-
2024-12-13
- CAS:177036-94-1
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 100kg
- Ambrisentan
-
- $30.00 / 5mg
-
2024-11-19
- CAS:177036-94-1
- Min. Order:
- Purity: 99.86%
- Supply Ability: 10g
- Ambrisentan
-

- $0.00 / 1g
-
2024-11-18
- CAS:177036-94-1
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
|
| | Ambrisentan Basic information |
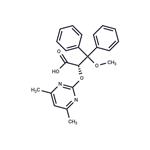
| Product Name: | Ambrisentan | | Synonyms: | BSF 208075,(+)-(2S)-2-[(4,6-DIMETHYLPYRIMIDIN-2-YL)OXY]-3-METHOXY-3,3-DIPHENYLPROPANOIC ACID;AMBRISENTAN;(aS)-a-[(4,6-Dimethyl-2-pyrimidinyl)oxy]--methoxy--phenylbenzenepropanoic Acid;BSF 208075;LU 208075;2-(4,6-Dimethylpyrimidin-2-yl)oxy-3-methoxy-3,3-di(phenyl)propanoic acid;(S)-2-(4,6-dimethylpyrimidin-2-yloxy)-3-methoxy-3,3-diphenylpropanoic acid;(+-)-(2S)-2-((4,6-Dimethylpyrimidin-2-yl)oxy)-3-methoxy-3,3-diphenylpropanoic acid,bsf-208075 | | CAS: | 177036-94-1 | | MF: | C22H22N2O4 | | MW: | 378.42 | | EINECS: | 658-059-9 | | Product Categories: | APIs;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Other APIs | | Mol File: | 177036-94-1.mol | 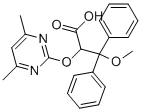 |
| | Ambrisentan Chemical Properties |
| Melting point | >150°C (dec.) | | Boiling point | 551.1±60.0 °C(Predicted) | | density | 1.228±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 0.97±0.10(Predicted) | | color | White to Off-White | | Merck | 14,384 | | InChI | InChI=1S/C22H22N2O4/c1-15-14-16(2)24-21(23-15)28-19(20(25)26)22(27-3,17-10-6-4-7-11-17)18-12-8-5-9-13-18/h4-14,19H,1-3H3,(H,25,26) | | InChIKey | OUJTZYPIHDYQMC-UHFFFAOYSA-N | | SMILES | C(OC1N=C(C)C=C(C)N=1)(C(O)=O)C(OC)(C1=CC=CC=C1)C1=CC=CC=C1 |
| | Ambrisentan Usage And Synthesis |
| Description | Ambrisentan is a selective endothelin-A (ETA) receptor antagonist introduced for the oral treatment of patients with pulmonary arterial
hypertension (PAH), to improve exercise capacity and delay clinical worsening.
It is the third ET-receptor antagonist to be marketed for this indication behind
bosentan and sitaxsentan. PAH is a rare disease of the small pulmonary arteries
characterized by vascular proliferation and remodeling, resulting in a
progressive increase in pulmonary vascular resistance and pulmonary arterial
pressure, and ultimately, right ventricular failure and premature death. Early symptoms of PAH include gradual onset of shortness of breath, fatigue,
palpitation, edema, and fainting. Endothelin-1 (ET-1), a potent vasoconstrictor
and smooth muscle mitogen, is a key contributor to the acceleration of the
disease, and its effects are mediated through activation ofETA and ETB
receptors. | | Description | Ambrisentan is a nonpeptide endothelin A (ETA) receptor antagonist (IC50s = 0.251, 0.316, 0.398, 251, and 630 nM for rat preparations of heart, bladder, kidney, lung, and cerebral cortex, respectively). It inhibits contraction of isolated rabbit aortic rings induced by endothelin-1 (ET-1; ) by 43.23% when used at a concentration of 1 μM. Ambrisentan inhibits ET-1-induced contraction of human pulmonary and radial arteries in vitro (Kd = 0.042 and 0.11 μM, respectively). In a rat model of neonatal hyperoxic lung injury, ambrisentan (20 mg/kg per day, s.c.) reduces pulmonary arterial hypertension (PAH) as well as decreases PAH-induced right ventricular hypertrophy (RVH) and peak RV pressure. Formulations containing ambrisentan have been used to treat PAH. | | Chemical Properties | White to Off White Solid | | Originator | Abbott (US) | | Uses | antihypertensive;endothelin receptor antagonist | | Uses | Nonpeptide endothelin ETA receptor antagonist. Antihypertensive | | Definition | ChEBI: Ambrisentan is a diarylmethane. | | Brand name | Letairis | | General Description | Ambrisentan, (+)-(2S)-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropanoic acid(Letairis), is a potent ETA selective endothelin antagonist that,is indicated, in the treatment of pulmonary arterial hypertension(PAH). PAH is a rare disease that if left untreated has ahigh mortality rate. In June of 2007, the FDA granted approvalof ambrisentan for once-daily treatment of PAH.Studies have shown that it improves a 6-minute walk by about30 to 60 m for patients receiving placebo. | | Clinical Use | Endothelin A (ETA) receptor antagonist:
Treatment of pulmonary arterial hypertension | | Synthesis | Both the discovery and process routes to the synthesis
of ambrisentan have been published and the process
route is described as shown in the scheme. Reacting a
mixture of benzophenone (14) and sodium methoxide in
THF at 0??C with methylchloroacetate over a four hour period
provided glycidate 15 which was taken forward without
purification to the subsequent step. Addition of ptoluenesulfonic
acid monohydrate to a solution of glycidate
15 in methanol was followed by heating at reflux and distilling
out the solvent until the temperature reached 66??C. While
the solution was still refluxing, 10% potassium hydroxide
was added and the remaining organic solvent was distilled
out until the temperature reached 94??C, providing complete
hydrolysis to acid 16. The reaction was cooled to room temperature
and diluted with water and methyl tert-butylether
(MTBE) then acidified with 10% sulfuric acid. The MTBE
layer was separated and taken to the next step. Additional
MTBE and methanol were added to the crude acid 17 and the
resulting mixture was heated at reflux. (S)-1-(4-chlorophenyl)
ethylamine was added to the refluxing solution and the
resulting mixture was allowed to cool to 0-5??C slowly at a
rate of 10??C/h which resulted in crystallization of the salt 19
in 33% overall yield from benzophenone and 99% e.e. The
chiral hydroxyl acid salt 19 was mixed with sulfone 20 and
lithium amide in a toluene/DMF mixture and heated at 45??C
for 12 hours to give, after acidic workup and crystallization,
ambrisentan (II) in 84% yield as a colorless powder with
99.8% e.e. 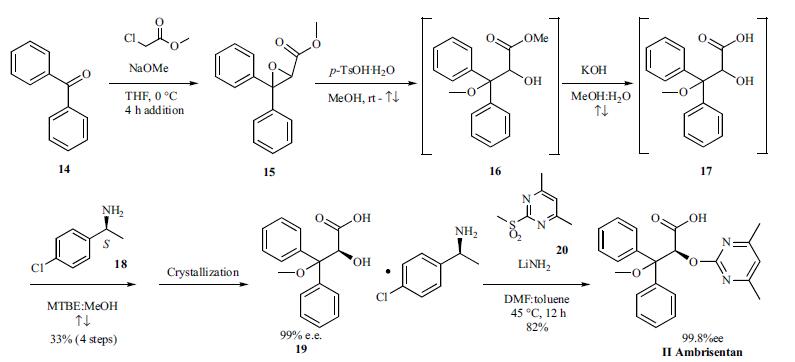
| | Drug interactions | Potentially hazardous interactions with other drugs
Ciclosporin: concentration of ambrisentan doubled
with an increased risk of side effects; maximum dose
5 mg daily. | | Metabolism | Ambrisentan is glucuronidated via several UGT
isoenzymes (UGT1A9S, UGT2B7S and UGT1A3S)
to form ambrisentan glucuronide (13%). Ambrisentan
also undergoes oxidative metabolism mainly by CYP3A4
and to a lesser extent by CYP3A5 and CYP2C19 to form
4-hydroxymethyl ambrisentan (which has little activity)
which is further glucuronidated to 4-hydroxymethyl
ambrisentan glucuronide.
Ambrisentan is excreted mainly by the liver, although the
contribution of hepatic metabolism and biliary excretion
is unknown. | | storage | Store at +4°C | | references | [1] vatter h, seifert v. ambrisentan, a non-peptide endothelin receptor antagonist. cardiovascular drug reviews, 2006, 24(1): 63-76.
[2] barst r j. a review of pulmonary arterial hypertension: role of ambrisentan. vascular health and risk management, 2007, 3(1): 11. |
| | Ambrisentan Preparation Products And Raw materials |
|