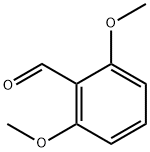
2,6-Dimethoxybenzaldehyde synthesis
- Product Name:2,6-Dimethoxybenzaldehyde
- CAS Number:3392-97-0
- Molecular formula:C9H10O3
- Molecular Weight:166.17
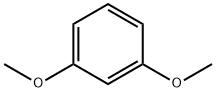
151-10-0
447 suppliers
$5.00/10g
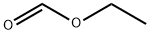
109-94-4
477 suppliers
$10.00/10g

3392-97-0
240 suppliers
$10.00/1g
Yield:3392-97-0 48%
Reaction Conditions:
Stage #1:1,3-Dimethoxybenzene with n-butyllithium in tetrahydrofuran;hexane at 0; for 2 h;
Stage #2:formic acid ethyl ester in tetrahydrofuran;hexane at -78; for 3 h;
Stage #3: with ethanol;iodine;potassium carbonate in tetrahydrofuran;hexane at -78 - 20; for 15 h;
Steps:
4.2. Typical procedure for one-pot conversion of aromatic bromides into aromatic ethyl esters with ethyl formate
General procedure: n-BuLi (1.67 M solution in hexane, 1.32 mL, 2.2 mmol) was added dropwise into a solution of p-bromochlorobenzene (383 mg, 2.0 mmol) in THF (3 mL) at -78 °C for 30 min. Then, ethyl formate (1.6 mL, 20 mmol) was added to the mixture and the obtained mixture was stirred at -78 °C. After 3 h at the same temperature, I2 (1523 mg, 6 mmol), K2CO3 (1382 mg, 10 mmol) and EtOH (3 mL) were added at -78 °C and the mixture was stirred for 14 h at rt. The reaction mixture was quenched with satd aq Na2SO3 (5 mL) and was extracted with CHCl3 (3×20 mL). The organic layer was washed with brine and dried over Na2SO4 to provide ethyl 4-chlorobenzoate in 77% yield. If necessary, the product was purified by short column chromatography (SiO2:hexane:EtOAc=9:1) to give pure ethyl 4-chloro-1-benzoate as a colorless oil.
References:
Ushijima, Sousuke;Moriyama, Katsuhiko;Togo, Hideo [Tetrahedron,2012,vol. 68,# 24,p. 4701 - 4709] Location in patent:experimental part
4885-02-3
184 suppliers
$10.00/1g

151-10-0
447 suppliers
$5.00/10g
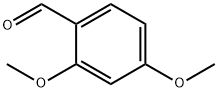
613-45-6
481 suppliers
$10.00/5g

3392-97-0
240 suppliers
$10.00/1g
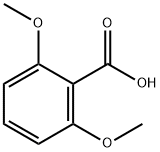
1466-76-8
269 suppliers
$5.00/25mg

3392-97-0
240 suppliers
$10.00/1g
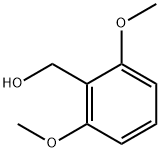
16700-55-3
69 suppliers
$59.00/1 g

3392-97-0
240 suppliers
$10.00/1g
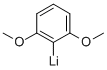
2785-97-9
5 suppliers
inquiry

3392-97-0
240 suppliers
$10.00/1g