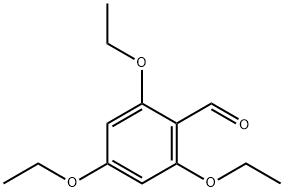
2,4,6-TRIETHOXYBENZALDEHYDE synthesis
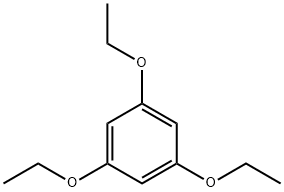
- Product Name:2,4,6-TRIETHOXYBENZALDEHYDE
- CAS Number:59652-88-9
- Molecular formula:C13H18O4
- Molecular Weight:238.28
Yield:59652-88-9 34%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 20 - 60;
Steps:
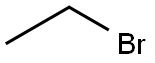
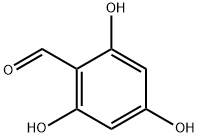
96 was synthesized in one step. The trihydroxide (1.88 g, 9.9 mmol) was dissolved in DMF. NaH (0.960 g, 0.024 mol, in 60% dispersion in mineral oil) was added, followed by EtBr (16.240 g, 11.05 mL, 0.149 mol). The mixture was stirred over night at room temperature. After no trialkylated product was observed, another batch of NaH (0.301 g, 0.0125 mol, in 60% dispersion in mineral oil) was added, together with more EtBr (2.05 g, 1.397 mL, 0.0188 mol). The mixture was stirred over night at 60°C and quenched with ?0 (50 mL). The crude product was extracted with DCM, and the organic phase was washed twice with saturated brine. Subsequently, the solvent was removed and the residual crude product was purified with column chromatography (Si02, 3:2 mixture of Hexane/EtOAc) to afford the clean product as a colorless solid. Yield: 0.970 g, 3.4 mmol, 34%, Rf = 0.21 NMR (CDCI3, 400 MHz): 10.34 (s, 1H, CO-H), 5.99 (s, 2H, aryl-H), 4.03 (q, 6H, CH2), 1.39 (m, 9H, CH3)l3C NMR (CDC13, 75 MHz): 187.9, 165.5, 163.5, 109.1. 91.6, 64.6, 63.9, 14.65HR-ESI-MS: m/z calcd for Ci3H1909: 239.1283. found: 239.1286 M+H+ Elemental Analysis: N: 0.07, C: 65.37, H: 7.48 (calcd. C: 65.53, H: 7.61)
References:
WO2011/100829,2011,A1 Location in patent:Page/Page column 105