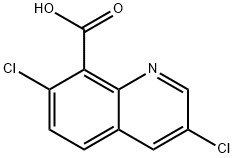
Quinclorac
|
|
Quinclorac Eigenschaften
- Schmelzpunkt:
- 274°C
- Siedepunkt:
- 405.4±40.0 °C(Predicted)
- Dichte
- 1.7500
- Brechungsindex
- 1.6100 (estimate)
- Flammpunkt:
- 100 °C
- storage temp.
- Sealed in dry,Room Temperature
- L?slichkeit
- DMSO (Slightly), Methanol (Slightly)
- Aggregatzustand
- Solid
- pka
- -3.26±0.10(Predicted)
- Farbe
- Pale Yellow
- BRN
- 7761858
- InChIKey
- FFSSWMQPCJRCRV-UHFFFAOYSA-N
- EPA chemische Informationen
- Quinclorac (84087-01-4)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xi | ||
|---|---|---|---|
| R-S?tze: | 43 | ||
| S-S?tze: | 2-24-37 | ||
| WGK Germany | 2 | ||
| RTECS-Nr. | VB1984000 | ||
| HS Code | 29334900 | ||
| Giftige Stoffe Daten | 84087-01-4(Hazardous Substances Data) |
| Bildanzeige (GHS) |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | ||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||
| Sicherheit |
|
Quinclorac Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R43:Sensibilisierung durch Hautkontakt m?glich.S-S?tze Betriebsanweisung:
S2:Darf nicht in die H?nde von Kindern gelangen.S24:Berührung mit der Haut vermeiden.
S37:Geeignete Schutzhandschuhe tragen.
Definition
ChEBI: A quinolinemonocarboxylic acid that is quinoline-8-carboxylic acid in which the hydrogens at positions 3 and 7 have been replaced by chlorines. It is used (particularly as its dimethylamine salt, known as quinclorac-dimethylammonium) as a (rather persisten ) herbicide for the post-emergence control of weeds in rice, grass and turf. It is not approved for use within the European Union.Pharmakologie
The mechanism of action of quinclorac is controversial. Koo et al. (27) reported that quinclorac inhibited the incorporation of glucose into the cell wall of maize root cells. The synthesis of cellulose as well as some hemicellulose was inhibited at concentrations that inhibited whole plant growth, leading to the conclusion that quinclorac inhibits cell expansion by inhibiting glucose incorporation into the cell wall (27). These authors found that quinclorac inhibited root elongation in sensitive grasses at concentrations that inhibited cell wall biosynthesis. Cell wall biosynthesis in tolerant grasses was much less affected by quinclorac compared with sensitive grasses (28). Grossmann, on the other hand, believes that quinclorac acts as an auxinic herbicide in grasses as well as broadleaf plants, and that its herbicidal effects are due to production of cyanide (which is a byproduct of ethylene biosynthesis) and induction of the plant hormone abscisic acid (29). In this scenario, inhibition of cell wall biosynthesis would be considered a side effect, and not the primary cause of herbicidal injury. Differences in tolerance to quinclorac in this case are solely due to differences in ethylene induction, in that tolerant plants do not respond to quinclorac by synthesizing ethylene (29,30).Stoffwechsel
Quinclorac has been under development by BASF since 1982, and it has been marketed since 1984, as the herbicide Facet. Synthesis data are not currently available. Absorption and uptake of quinclorac is rapid, with 85% uptake in crabgrass within the first 30 minutes. Although uptake of quinclorac is rapid, translocation is generally poor, especially in sensitive plants. Tolerant species, such as Kentucky bluegrass, tend to transport more of the chemical away from the area of uptake. This may, in part, explain how tolerant plants are less affected by quinclorac. Quinclorac is metabolized very slowly in both sensitive and tolerant grass species. In leafy spurge, themajor metabolite found 7 days after foliar application of quinclorac was a pentosylglucose ester.In susceptible grasses, early symptoms of quinclorac activity include rapid chlorosis starting at the elongation zone of newly expanding leaves. This is followed by more widespread chlorosis and, eventually, necrosis. On the other hand, quinclorac seems to affect susceptible broadleaf plants as an auxinic herbicide. Symptoms in broadleaf plants begin with induction of ethylene biosynthesis and epinastic bending of shoots and leaves. This is followed by growth inhibition, chlorosis, wilting, and, finally, necrosis.
Quinclorac Upstream-Materialien And Downstream Produkte
Upstream-Materialien
2-Nitrotoluol
2-toluidine
3-Chlor-o-toluidin
Glycerol
Eisentrichlorid
7-(Dimethylamino)-4-methyl-2-benzopyron
Salpetersure
Celite
2-methylnitrobenzene
Downstream Produkte
Quinclorac Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 319)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8810 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5857 | 58 |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 |
aroma@qdtrustagri.com | China | 301 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20284 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29880 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 |
sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
84087-01-4()Verwandte Suche:
Pyridin-2,3-dicarbonsure
Isochinolin
Ethoxychin
4,7-Dichlorchinolin
ACETIC ACID
8-Hydroxychinolin
Folsure
2-Chlorchinolin
3-(2,2-Dichlorvinyl)-2,2-dimethylcyclopropancarbonsure
3,6-Dichlorpyridin-2-carbonsure
2,4-Dichloroquinazoline
2,3-Dichlorchinoxalin
2,6-Dichloroquinoxaline
Halquinol
8-Methylchinolin
Chinolin
Quinclorac
CARBOXYLIC ACID
- 3,7-Dichlorchinolin-8-carbonsure
- QUINCHLORAC (3,7-DICHLOR- 8-QUINOLINECARBOXYLIC ACID)
- 8-Quinolinecarboxylicacid,3,7-dichloro-
- bas51400h
- Drive
- BAS-51406-H
- quincloractech
- BAS 514
- 3,7-DICHLORO-8-QUINOLINECARBOXYLIC ACID
- 3,7-Dichloroquinoline acid-8
- Dichloroquinolinic acid W.P.
- Quinclorac W.P.
- 3,4-Dichloro-8-quinoline carboxylic acid
- Quinclorac 500mg [84087-01-4]
- Dichloro-8-quinolinecarboxylic acid
- 7-Dichloro-8-quinolinecarboxylic acid (Quinchlorac)
- Facet LA
- ParMount 75WG
- Paramount
- Parmount
- Quinclorac 0.1
- facet 75 df
- FACET(R)
- FAS-NOX
- BAS-514-H
- QUEEN
- QUINCLORAC
- QUINCHLORAC PESTANAL (3,7-DICHLOR- 8-QUI
- 3,7-DICHLORO-8-QUINOLINECARBOXYLIC ACID (QUINCHLORAC)
- 3,7-Dichloro-8-quinolinecarbox
- quinclorac (bsi,iso)
- QUINCLORACE
- 3,7-DICHLORO-8-CARBOXYLICACID
- Quinclorac Solution, 1000ppm
- Quinchlorac approx
- 3,7-Dichloroquinoline-8-carboxylic acid Solution
- Quinclorac Standard
- Quinclorac@100 μg/mL in AcCN
- Quinclorac @100 μg/mL in Methanol
- QuincloracSolution,1,000mg/L,1ml
- FACET
- 3,7-DICHLOROQUINOLINE-8-CARBOXYLIC ACID
- dichloroquinolinicacid
- QUINCHLORAC
- 3,7-dichloro-8-quinolinecarboxylicaci
- Quinclorac in Acetone
- Quinclorac 10.0 μg/ml Acetonitrile
- Quinclorac 100 μg/ml Acetone
- 84087-01-4
- C10H5Cl2MP2
- C10H5CLNO2
- HERBICIDE