4-Acryloylmorpholine Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.
R41:Gefahr ernster Augensch?den.
R43:Sensibilisierung durch Hautkontakt m?glich.
R48/22:Gesundheitssch?dlich: Gefahr ernster Gesundheitssch?den bei l?ngerer Exposition durch Verschlucken.
S-S?tze Betriebsanweisung:
S23:Gas/Rauch/Dampf/Aerosol nicht einatmen(geeignete Bezeichnung(en) vom Hersteller anzugeben).
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Beschreibung
4-Acryloylmorpholine (4-AM) is a water-soluble monomer, an acrylamide derivative containing a heterocyclic tetrahydrooxazine substituent. Homo- and copolymers of 4-АМ have several valuable properties. They are soluble both in water and in many organic solvents such as alcohols, chloroform, tetrahydrofuran, and dioxane, are non-toxic, and are used for solid-phase synthesis of peptides in chromatography, as materials for composite semipermeable membranes, in catalysis, and for biomedical purposes. Various functional groups are introduced into poly-4-AM to expand its application fields. Random copolymers of 4-АМ with carboxylic acids (acrylic, 4-pentenoic, undecenoic, 2-acrylamido-2-methyl-1-propanesulfonic), behaving as anionic polyelectrolytes, and copolymers with N-hydroxysuccinimide ester of acrylic acid, acting as polymeric carriers, have been synthesized[2-3].
Chemische Eigenschaften
4-Acryloylmorpholine has a melting point of ?35 °C and is clear liquid at room
temperature. At 25 °C, it has a density of 1.122 g/mL. It needs to be
stored at 2-8°C.
Verwenden
4-Acryloylmorpholine is used in adhesives, UV curable resins, industrial coatings, UV printing ink, oil recovery polymer, medicinal and commodity chemicals.
Reaktionen
Using cobalt catalysis, diethylzinc promotes the conjugate reduction of 4-acryloylmorpholine to produce the corresponding ethylzinc enolate, which reacts with N-tosyl aldimines to afford β-aminoamides
[1].
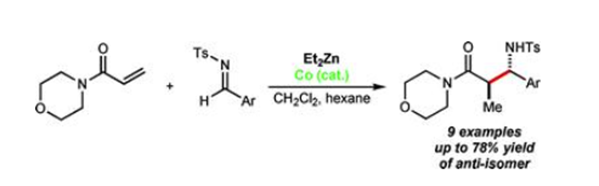
Synthese
A
solution of 0.04 mol of the corresponding amine in 20 ml of anhydrous
methylene chloride was slowly added at 0-5??C to 0.02 mol of acryloyl
chloride in 20 ml of anhydrous methylene chloride. The mixture was
stirred for 3 h at room temperature in an inert atmosphere, and the
precipitate was filtered off and washed with methylene chloride (2 ?á 10
ml). The organic layer was washed in succession with 5 ml of water and 5
ml of a saturated solution of NaHCO3 and dried over Na2SO4, the solvent
was removed under reduced pressure, and the residue was purified by
column chromatography on silica gel using hexane-ethyl acetate (5 : 1 to
1 : 1) as eluent. 4-Acryloylmorpholine, Yield 1.78 g (63%).
IR spectrum, |í, cm-1: 2857, 1647, 1612, 1439, 1263, 1238, 1115, 1038,
953. 1H NMR spectrum, |?, ppm: 3.51-3.73 m (8H, NCH2CH2O),
5.72 d.d (1H, 3-Hcis, 3J = 10.6, 2J = 1.9 Hz), 6.29 d.d (1H, 3-Htrans,
3J = 16.7, 2J = 1.9 Hz), 6.57 d.d (1H, 2-H, J = 16.7, 10.6 Hz). 13C NMR
spectrum, |?C, ppm: 41.74 and 45.66 (CH2N), 66.22 (CH2O), 126.64 (C2),
127.69 (C3), 164.92 (C1). Mass spectrum, m/z (Irel, %): 141 (36) [M]+,
140 (12), 126 (58), 112 (22), 111 (15), 110 (15), 109 (12), 98 (10), 96
(26), 86 (72), 83 (13), 70 (14), 68 (14), 57 (17), 56 (86), 55 (100),
42 (23).
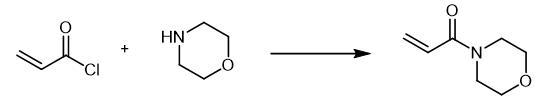
Fig. The synthetic method 2 of 4-Acryloylmorpholine
4-Acryloylmorpholine Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

