Kristallviolett (C.I. 42555) Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R52/53:Sch?dlich für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R50/53:Sehr giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R45:Kann Krebs erzeugen.
R41:Gefahr ernster Augensch?den.
R22:Gesundheitssch?dlich beim Verschlucken.
R40:Verdacht auf krebserzeugende Wirkung.
R34:Verursacht Ver?tzungen.
R10:Entzündlich.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R23/25:Giftig beim Einatmen und Verschlucken.
S-S?tze Betriebsanweisung:
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
S60:Dieses Produkt und sein Beh?lter sind als gef?hrlicher Abfall zu entsorgen.
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S46:Bei Verschlucken sofort ?rztlichen Rat einholen und Verpackung oder Etikett vorzeigen.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S39:Schutzbrille/Gesichtsschutz tragen.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S16:Von Zündquellen fernhalten - Nicht rauchen.
S22:Staub nicht einatmen.
Beschreibung
Crystal Violet is light sensitive. May react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release gaseous hydrogen.
Chemische Eigenschaften
dark green powder or crystals
Occurrence
Gentian is a fl owering perennial found in Europe and Asia.
Verwenden
Crystal Violet is used as an acid-base indicator, an alcohol denaturant, a biological stain, a textile dye and an indicator for copper salts. It is a component of gram staining in microorganisms and cell lines.
synthetische
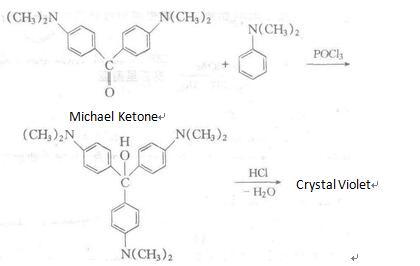
It is manufactured through using N, N-dimethylaniline as raw materials, followed by condensation, addition, chlorination and other reactions. Alternatively, it can be synthesized through the reaction between Michler ketone and N, N-dimethylaniline reaction in the presence of phosphorus oxychloride, followed by azeotropic reaction with hydrochloric acid.
Recrystallization in hot water will generate compound containing nine crystal water molecules.
Essential oil composition
A number of bitter compounds present in gentian are primarily amarogentin (strongly bitter), gentiopricin
(approximately 1.5% in fresh root), swertiamarin and gentiopricroside. The leaves and flowers contain mainly xanthones.
Secoiridoids and flavonoids were also detected. In the phase of flowering, leaves are rich with compounds possessing C-glycoside
structures while O-glycoside structures accumulate mainly before flowering.
Allgemeine Beschreibung
It belongs to triamino-triphenylmethane synthetic dyes; alkaline. It is an important dye used in bacterial Gram stain. The mixed reagent of crystal violet chloride and iodine is known as gentian violet.
Air & Water Reaktionen
Insoluble in water.
Brandgefahr
Flash point data for Crystal Violet are not available, however, Crystal Violet is probably combustible.
Clinical Use
Gentian violet is variously known as hexamethyl-p-rosanilinechloride, crystal violet, methyl violet, and methylrosanilinechloride. It occurs as a green powder or greenflakes with a metallic sheen. The compound is soluble inwater (1:35) and alcohol (1:10) but insoluble in nonpolar organicsolvents. Gentian violet is available in vaginal suppositoriesfor the treatment of yeast infections. It is also used asa 1% to 3% solution for the treatment of ringworm and yeastinfections. Gentian violet has also been used orally as an anthelminticfor strongyloidiasis (threadworm) and oxyuriasis.
Sicherheitsprofil
Poison by ingestion,
intravenous, and intraperitoneal routes, An
experimental teratogen. Other experimental
reproductive effects. A human sktn irritant.
Human mutation data reported.
Questionable carcinogen with experimental
carcinogenic data. When heated to
decomposition it emits very toxic fumes of
NO, and Cl-.
l?uterung methode
Crystallise the dye from water (20mL/g), the crystals being separated from the chilled solution by centrifugation, then wash them with chilled EtOH (solubility is 1g in 10 mL of hot EtOH) and diethyl ether and dry under vacuum. It is soluble in CHCl3 but insoluble in Et2O. The carbinol is precipitated from an aqueous solution of the dye-hydrochloride, using excess NaOH, then dissolve in HCl and recrystallise it from water as the chloride [UV and kinetics: Turgeon & La Mer J Am Chem Soc 74 5988 1952]. The carbinol base has m 195o (needles from EtOH). The diphthalate (blue and turns red in H2O) crystallises from H2O, m 153-154o(dec at 185-187o)[Chamberlain & Dull J Am Chem Soc 50 3089 1928]. [Beilstein 13 H 233, 13 IV 2284.]
Kristallviolett (C.I. 42555) Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

