55726-47-1
中文名稱
依諾他濱
英文名稱
Enocitabine
CAS
55726-47-1
分子式
C31H55N3O6
MDL 編號
MFCD00866294
分子量
565.78
MOL 文件
55726-47-1.mol
更新日期
2023/03/20 15:41:19
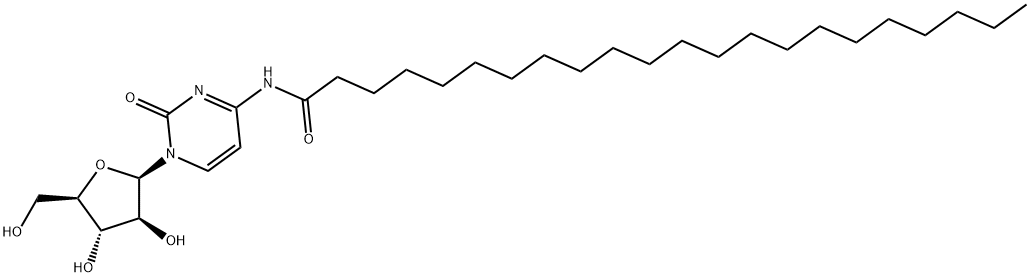 55726-47-1 結(jié)構(gòu)式
55726-47-1 結(jié)構(gòu)式
基本信息
中文別名
依諾他濱山崳阿糖啶
山崳阿糖嘧啶
N-[1-[3,4-二羥基-5-(羥甲基)氧雜環(huán)戊-2-基]-2-氧代嘧啶-4-基]山崳酸酰胺
英文別名
BEHENOYLCYTOSINE ARABINOSIDEBH-AC
ENOCITABINE
N-(1-BETA-D-ARABINOFURANOSYL-1,2-DIHYDRO-2-OXO-4-PYRIMIDINYL)DOCOSANAMIDE
NSC-239336
SUNRABIN
n-(1-beta-d-arabinofuranosyl-1,2-dihydro-2-oxo-4-pyrimidinyl)-docosanamid
n(sup4)-behenoyl-1-beta-d-arabinofuranosylcytosine
n(sup4)-behenoylcytosinearabinoside
ENOCITABINE 98.5+% ANTINEOPLASTIC DRUGS
N-(1-B-D-Arabinofuranosyl-1,2-dihydro-2-oxo-4-pyrimidinyl)docosanamide, Behenoylcytosine Arabinoside, BH-AC, NSC-239336, Sunrabin
Docosanamide, N-(1-β-D-arabinofuranosyl-1,2-dihydro-2-oxo-4-pyrimidinyl)-
N4-Behenoyl-1-β-D-arabinofuranosylcytosine
N4-Behenoylcytosine arabinoside
N-[1-[3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxopyrimidin-4-yl]docosanamide
N-[1-[(2R,3S,4R,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxo-pyrimidin-4-yl]docosanamide
1-β-D-Arabinofuranosyl-4-(1-oxodocosyl)amino-1,2-dihydropyrimidin-2-one
所屬類別
生物化工:激動劑抑制劑物理化學(xué)性質(zhì)
熔點141-142°C
比旋光度D +70° (c = 1 in THF, 22°)
沸點630.87°C (rough estimate)
密度1.0967 (rough estimate)
折射率1.7800 (estimate)
儲存條件Keep in dark place,Sealed in dry,Store in freezer, under -20°C
溶解度DMSO (Slightly, Heated), Methanol (Slightly, Heated), THF (Slightly, Heated, Soncited)
酸度系數(shù)(pKa)10.14±0.20(Predicted)
形態(tài)固體
顏色白色至灰白色
CAS 數(shù)據(jù)庫55726-47-1(CAS DataBase Reference)
依諾他濱價格(試劑級)
| 報價日期 | 產(chǎn)品編號 | 產(chǎn)品名稱 | CAS號 | 包裝 | 價格 |
| 2025/02/08 | HY-123523 | 依諾他濱 Enocitabine | 55726-47-1 | 1 mg | 1523元 |
| 2025/02/08 | HY-123523 | 依諾他濱 Enocitabine | 55726-47-1 | 5mg | 3050元 |
| 2025/02/08 | HY-123523 | 依諾他濱 Enocitabine | 55726-47-1 | 10mg | 4280元 |
常見問題列表
生物活性
Enocitabine 是一種核苷類似物,是一種有效的 DNA 復(fù)制抑制劑和 DNA鏈終止劑。Enocitabine 抑制人巨細(xì)胞病毒的復(fù)制,具有抗白血病和抗病毒活性。靶點
DNA replication; CMV
體外研究
Enocitabine is resistant to deamination because Enocitabine bears a highly lipophilic group at the 4-amino position of the cytosine moiety of cytarabine.
The combined effects of Pirarubicin and Enocitabine on HeLa S3 human uterine cervix carcinoma and K562 human myelocytic leukemia cells are determined by enhancement of their cytotoxic activities. Enocitabine or etoposide shows synergistic effects on HeLa S3 and K562 cells.
In the presence of Enocitabine, triphosphate forms of the nucleoside analogs are detected in the human cytomegalovirus (HCMV)-infected cells, and synthesis of HCMV DNA is strongly suppressed.