| Identification | More | [Name]
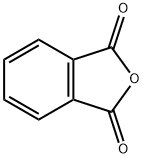
Phthalic anhydride | [CAS]
85-44-9 | [Synonyms]
1,2-BENZENEDICARBONIC ACID, ANHYDRIDE
1,2-BENZENE DICARBOXYLIC ACID ANHYDRIDE
1,3-DIOXOPHTHALAN
1,3-DIOXOPHTHALANE
1,3-ISOBENZOFURANDIONE
1,3-ISOBENZOFURANIDONE
AKOS BBS-00004337
O-PHTHALIC ANHYDRIDE
PHTHALANDIONE
PHTHALIC ACID ANHYDRIDE
PHTHALIC ANHYDRIDE
1,2-Benzenedicarboxylic Anhydride
1,2-benzenedicarboxylicanhydride
1,3-dihydro-1,3-dioxo-isobenzofura
1,3-dihydro-1,3-dioxoisobenzofuran
1,3-phthalandion
1,3-Phthalandione
2-Benzofuran-1,3-dione
Anhydrid kyseliny ftalove
Anhydride phtalique | [EINECS(EC#)]
201-607-5 | [Molecular Formula]
C8H4O3 | [MDL Number]
MFCD00005918 | [Molecular Weight]
148.12 | [MOL File]
85-44-9.mol |
| Chemical Properties | Back Directory | [Appearance]
Phthalic Anhydride is moderately flammable, white solid (flake) or a clear, colorless, mobile liquid (molten) Characteristic, acrid, choking odor | [Melting point ]
131-134 °C(lit.)
| [Boiling point ]
284 °C(lit.)
| [bulk density]
500-700kg/m3 | [density ]
1,53 g/cm3 | [vapor density ]
5.1 (vs air)
| [vapor pressure ]
<0.01 mm Hg ( 20 °C)
| [refractive index ]
1.4500 (estimate) | [Fp ]
152 °C
| [storage temp. ]
Store at RT. | [solubility ]
6g/l (slow decomposition) | [form ]
Flaky Crystals | [pka]
2.97[at 20 ℃] | [color ]
White | [Odor]
Characteristic choking odor | [PH]
2 (6g/l, H2O, 20℃) | [PH Range]
2 at 6 g/l at 20 °C | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents, strong bases, moisture, strong acids. Dust may form an explosive mixture with air. | [explosive limit]
1.7-10.5%(V) | [Water Solubility ]
6 g/L (20 ºC) | [Sensitive ]
Moisture Sensitive | [Merck ]
14,7372 | [BRN ]
118515 | [Henry's Law Constant]
6.29 at 20 °C (approximate - calculated from water solubility and vapor pressure) | [Exposure limits]
NIOSH REL: TWA 6 mg/m3 (1 ppm), IDLH 60 mg/m3; OSHA PEL: TWA 12
mg/m3 (2 ppm); ACGIH TLV: TWA 1 ppm (adopted). | [Contact allergens]
Phthalic anhydride is used in the manufacture of unsaturated
polyesters and as a curing agent for epoxy resins.
When used as a pigment, it can be responsible for sensitization
in ceramic workers. Phthalic anhydride per se is
not responsible for the sensitization to the resin used in
nail varnishes phthalic anhydride/trimellitic anhydride/
glycols copolymer. | [LogP]
2.07 at 20℃ | [CAS DataBase Reference]
85-44-9(CAS DataBase Reference) | [NIST Chemistry Reference]
Phthalic anhydride(85-44-9) | [EPA Substance Registry System]
85-44-9(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline solid with choking odour | [Uses]
Phthalic Anhydride is an organic compound and the anhydride of phthalic acid (P384480). Phthalic Anhydride is an important industrial chemical commonly used in large-scale production of plasticizers f
or plastics. Recent research have also evaluated Phthalic Anhydride as potential antibacterial agent. | [Definition]
ChEBI: The cyclic dicarboxylic anhydride that is the anhydride of phthalic acid. | [Uses]
manufacture of phthaleins, phthalates, benzoic acid, synthetic indigo, artificial resins (glyptal). | [General Description]
A colorless to white lustrous solid in the form of needles with a mild distinctive odor. Moderately toxic by inhalation or ingestion and a skin irritant. Melting point 64°F Flash point 305°F. Forms a corrosive solution when mixed with water. Used in the manufacture of materials such as artificial resins. | [Reactivity Profile]
PHTHALIC ANHYDRIDE(85-44-9) reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Undergoes exothmeric nitration with fuming nitric acid-sulfuric acid and may give mixtures of the potentially explosive phthaloyl nitrates or nitrites or their nitro derivatives [Chem. & Ind. 20:790. 1972]. PHTHALIC ANHYDRIDE(85-44-9) reacts violently with CuO at elevated temperatures [Park, Chang-Man, Richard J. Sheehan. hthalic Acids and Other Benzenepolycarboxylic Acids Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2005]. Mixtures of PHTHALIC ANHYDRIDE(85-44-9) and anhydrous CO2 explode violently if heated [eaflet No. 5, Inst. of Chem., London, 1940]. | [Air & Water Reactions]
Reacts, usually slowly with water to form phthalic acid and heat [Merck 11th ed. 1989]. The phthalic acid is somewhat soluble in water. | [Health Hazard]
Solid irritates skin and eyes, causing coughing and sneezing. Liquid causes severe thermal burns. | [Potential Exposure]
Phthalic anhydride is used in plasticizers; in the manufacture of phthaleins; benzoic acid; alkyd and polyester resins; synthetic indigo; and phthalic acid;which is used as a plasticizer for vinyl resins. To a lesser extent, it is used in the production of alizarin, dye, anthranilic acid; anthraquinone, diethyl phthalate; dimethyl phthalate; erythrosine, isophthalic acid; methylaniline, phenolphthalein, phthalamide, sulfathalidine, and terephthalic acid. It has also found uses as a pesticide intermediate. | [Fire Hazard]
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water, or milk. Do not induce vomiting. | [Shipping]
UN2214 Phthalic anhydride with>.05 % maleic anhydride, Hazard class: 8; Labels: 8-Corrosive material. | [Incompatibilities]
Dust forms an explosive mixture with air. Phthalic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Converted to phthalic acid in hot water. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. caustics, ammonia, amines, water. Reacts violently with copper oxide or sodium nitrite 1 heat. | [Description]
Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. This colourless solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics.

Phthalic anhydride is an important chemical intermediate in the plastics industry from which are derived numerous phthalate esters that function as plasticizers in synthetic resins. Phthalic anhydride itself is used as a monomer for synthetic resins such as glyptal, the alkyd resins, and the polyester resins.
Phthalic anhydride is also used as a precursor of anthraquinone, phthalein, rhodamine, phthalocyanine, fluorescein, and xanthene dyes.
Phthalic anhydride is used in the synthesis of primary amines, the agricultural fungicide phaltan, and thalidomide. Other reactions with phthalic anhydride yield phenolphthalein, benzoic acid, phthalylsulfathiazole (an intestinal antimicrobial agent), and orthophthalic acid.
| [Waste Disposal]
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
| [Physical properties]
Colorless to pale cream crystals with a characteristic, choking odor. Moisture sensitive. Odor
threshold concentration is 53 ppb (quoted, Amoore and Hautala, 1983). | [Preparation]
The most important modifying component used in the
manufacture of linear unsaturated polyesters is phthalic anhydride. The
anhydride is generally obtained by the oxidation of o-xylene:
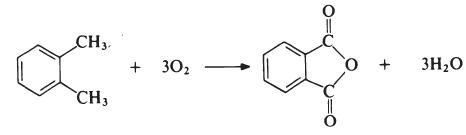
The reaction is carried out in the vapour phase by passing a mixture of
o-xylene and air over a catalyst such as vanadium pentoxide supported on
silica and promoted with titanium dioxide at about 400??C. The exit gases are
cooled and the phthalic anhydride is collected and purified by distillation
under reduced pressure. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 25, p. 616, 1960 DOI: 10.1021/jo01074a035
Synthesis, p. 612, 1973
Tetrahedron Letters, 20, p. 2301, 1979 DOI: 10.1016/S0040-4039(01)93957-7 | [Pharmaceutical Applications]
Phthalic anhydride reacted with cellulose acetate forms cellulose acetate phthalate (CAP), a common enteric coating excipient that has also been shown to have antiviral activity. Phthalic anhydride is a degradation product of CAP. | [Synthesis]
Phthalic anhydride is a precursor to a variety of reagents useful in organic synthesis. Important derivatives include phthalimide and its many derivatives. Chiral alcohols form half-esters (see above), and these derivatives are often resolvable because they form diastereomeric salts with chiral amines such as brucine. A related ring - opening reaction involves peroxides to give the useful peroxy acid:
C6H4(CO)2O + H2O2 → C6H4(CO3H)CO2H. | [Environmental Fate]
Chemical/Physical. Reacts with water to form o-phthalic acid (Kollig, 1993; Windholz et al.,
1983). Based on an observed rate constant of 7.9 x 10-9/sec, the hydrolysis half-life is 88 sec
(Hawkins, 1975).
Pyrolysis of phthalic anhydride in the presence of polyvinyl chloride at 600 °C for 10 min gave
the following compounds: biphenyl, fluorene, benzophenone, 9-fluorenone, o-terphenyl, 9-phenylfluorene,
and three unidentified compounds (Bove and Dalven, 1984). | [Purification Methods]
Distil the anhydride under reduced pressure. Purify it from the acid by extracting with hot CHCl3, filtering and evaporating. The residue is crystallised from CHCl3, CCl4 or *benzene, or sublimed. Fractionally crystallise it from its melt. Dry it under vacuum at 100o. [Saltiel J Am Chem Soc 108 2674 1986, Beilstein 17/11 V 253.] | [Precursor to dyestuffs]
Phthalic anhydride is widely used in industry for the production of certain dyes. A well-known application of this reactivity is the preparation of the anthroquinone dye quinizarin by reaction with parachloro phenol followed by hydrolysis of the chloride. | [Preparation of phthalate esters]
As with other anhydrides, the alcoholysis reaction is the basis of the manufacture of phthalate esters, which are widely used (and controversial - see endocrine disruptor) plasticizers. In the 1980s, approximately 6.5×109 kg of these esters were produced annually, and the scale of production was increasing each year, all from phthalic anhydride. The process begins with the reaction of phthalic anhydride with alcohols, giving the monoesters:
C6H4(CO)2O + ROH → C6H4(CO2H)CO2R
The second esterification is more difficult and requires removal of water:
C6H4(CO2H)CO2R + ROHC6H4(CO2R)2 + H2O
The most important di ester is bis (2-ethyl hexyl) phthalate ("DEHP"), used in the manufacture of polyvinyl chloride. | [Toxicity evaluation]
Phthalic anhydride modulates lipid mediator release and
cytokine formation and has sensitizing effects on the
respiratory tract. The local irritating effect particularly on the
mucous membranes probably depends on the hydrolysis to
phthalic acid. | [Toxics Screening Level]
The initial threshold screening level (ITSL) for phthalic anhydride (CAS # 85-44-9) is 20 μg/m 3
with an annual averaging time. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R37/38:Irritating to respiratory system and skin .
R41:Risk of serious damage to eyes.
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S24/25:Avoid contact with skin and eyes .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection .
S46:If swallowed, seek medical advice immediately and show this container or label .
S22:Do not breathe dust . | [OEB]
B | [OEL]
TWA: 6 mg/m3 (1 ppm) | [RIDADR ]
2214 | [WGK Germany ]
1
| [RTECS ]
TI3150000
| [F ]
10-21 | [Autoignition Temperature]
580 °C | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29173500 | [Safety Profile]
Poison by ingestion.
Experimental teratogenic effects. A
corrosive eye, skin , and mucous membrane
irritant. A common air contaminant.
Combustible when exposed to heat or flame; can react with oxidzing materials.
Moderate explosion hazard in the form of
dust when exposed to flame. The
production of ths material has caused many
industrial explosions. Mixtures with copper
oxide or sodium nitrite explode when
heated. Violent reaction with nitric acid +
sulfuric acid above 80℃. To fight fire, use
CO2, dry chemical. Used in plasticizers,
polyester resins, and alkyd resins, dyes, and
drugs. See also ANHYDRIDES. | [Hazardous Substances Data]
85-44-9(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 1530 mg/kg LD50 dermal Rabbit > 3160 mg/kg | [IDLA]
60 mg/m3 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Benzene-->Maleic anhydride-->Naphthalene-->o-Xylene-->Vanadium(V) oxide-->Motor benzol-->Lubricating oil-->Technical naphthalene-->Storage sump | [Preparation Products]
2,3,4,5-Tetrafluorobenzoic acid-->2-Methyl anthraquinone-->2-Bibenzylcarboxylic acid-->2-Benzoylbenzoic acid-->DIPHENYL PHTHALATE-->1,2,5-OXADIAZOLE-3-CARBOXYLIC ACID-->CYCLOOCTENE-->Fluorescein-->DI-N-OCTYL PHTHALATE-->Dicapryl Phthalate-->3-METHYL-1,2,5-OXADIAZOLE-->Amino resin varnish-->Indobufen-->Folpet-->Didecyl phthalate-->2',7'-dibromo-3',6'-dihydroxyspiro[isobenzofuran-1(3H),9'-[9H]xanthene]-3-one-->Diallyl phthalate-->Alkyd resin insulating paint-->N-(Hydroxymethyl)phthalimide-->2-(MORPHOLINE-4-CARBONYL)-BENZOIC ACID-->N-(2-Bromoethyl)phthalimide-->2,3-DIHYDRO-2-PHENYL-1H-ISOINDOL-1-OXO-ISOINDOLINE-->DI-ISO-DECYL PHTHALATE-->Diisobutyl phthalate-->2-Ethylanthraquinone-->2-Chloroanthraquinone-->5-(2-Carboxybenzoyl)-2-chlorobenzenesulfonyl chloride-->DI-N-PENTYL PHTHALATE-D4-->Dicyclohexyl phthalate-->BENZALPHTHALIDE-->C.I. 564100-->2-BENZOYLBENZOYL CHLORIDE-->N-PHENYLPHTHALIMIDE-->Diisononyl phthalate-->1-CHLORO-4-METHOXYPHTHALAZINE-->Potassium hydrogen phthalate-->N-PHTHALOYL-DL-GLUTAMIC ANHYDRIDE-->Solvent Red 49-->Dinonyl phthalate-->Diisooctyl phthalate |
|
|